An Indispensable Link in Tumor Treatment: Cell Therapy
Cell therapy refers to the transplantation or infusion of normal or bioengineered human cells into a patient’s body. The newly infused cells can replace damaged cells or have stronger immune killing capabilities, thereby achieving the purpose of treating diseases. Cell therapy can be divided into stem cell therapy and immune cell therapy according to the type of cells. The types of stem cells used for clinical treatment mainly include bone marrow stem cells, hematopoietic stem cells, neural stem cells, skin stem cells, islet stem cells, adipose stem cells, etc. Stem cell therapy uses the differentiation and repair principles of human stem cells, transplanting healthy stem cells into the patient's body to repair diseased cells or rebuild normal cells and tissues. Immune cell therapy collects immune cells from the human body and cultivates them outside the body, making their number increase by thousands of times or modifying the immune cells to become targeted, more lethal cells. These cells are then re-infused into the human body to kill pathogens, cancer cells, and mutated cells in the blood and tissues, breaking immune tolerance, activating, and enhancing the body's immune ability.
With the listing and application of CAR-T cell therapy, immune cell therapy is playing an irreplaceable role in tumor treatment thanks to its unique advantages. CAR-NK cell therapy can overcome some limitations of CAR-T, such as graft versus host disease and cytokine release syndrome. Due to its reduced toxicity and the advantage of easily preparing universal products, CAR-NK therapy is considered a rising star among cell products. CAR-M cell therapy attacks tumors in various ways by combining innate and adaptive immune systems. Meanwhile, TCR-T cell therapy has shown very encouraging results in solid tumors. In conclusion, as a hot area in tumor treatment, immune cell therapy showcases great potential and a bright future and is expected to bring good news to more tumor patients.
Cell Therapy Competitive Landscape
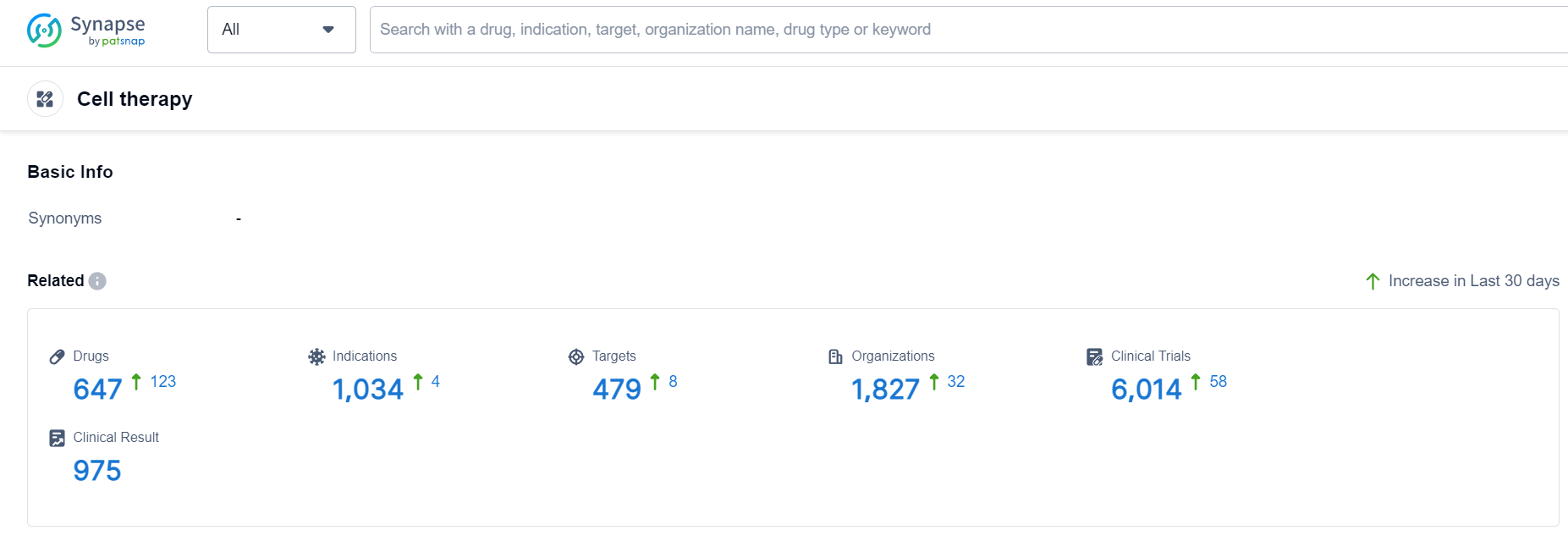
According to Patsnap Synapse, as of 19 Sep 2023, there are a total of 647 Cell therapy drugs worldwide, from 1827 organizations, covering 479 targets, 1034 indications, and conducting 6014 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this drug type.
Approved Cell Therapy Medicinal: Relmacabtagene Autoleucel
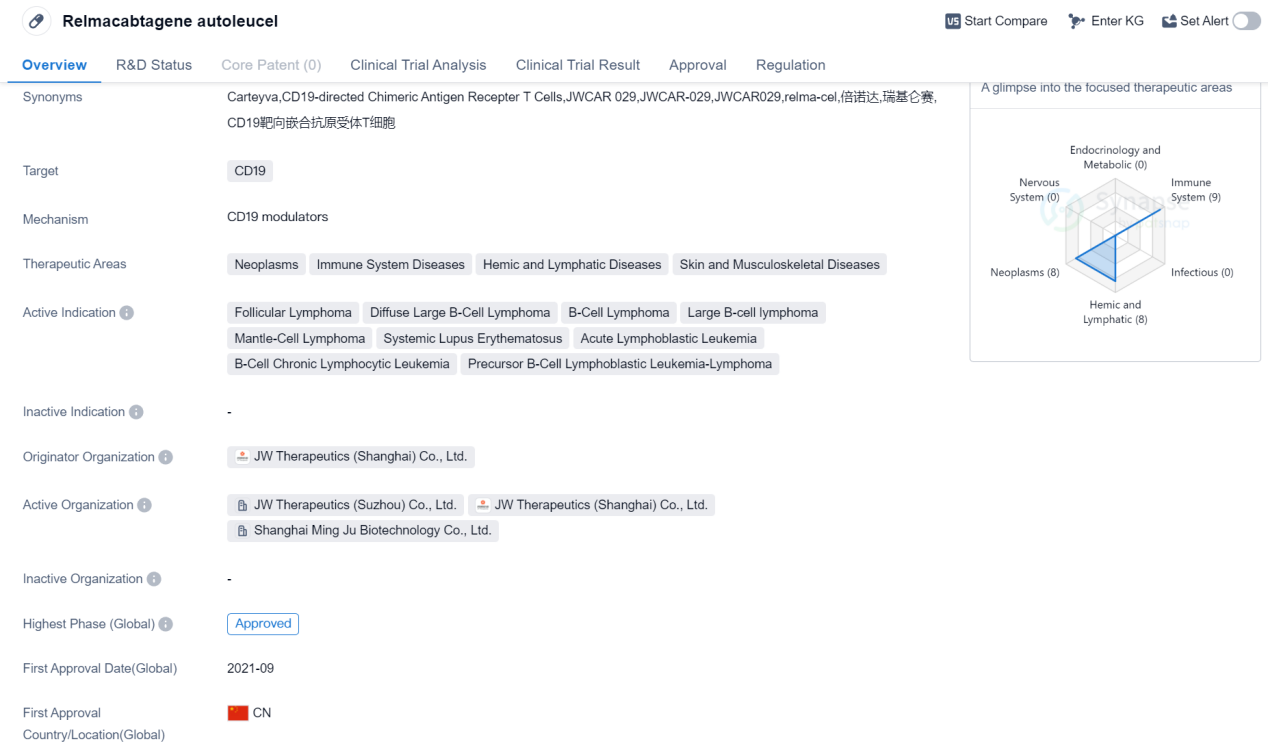
Relmacabtagene autoleucel is a CAR-T therapy that targets CD19, a protein found on the surface of certain cancer cells. It falls under the therapeutic areas of neoplasms, immune system diseases, hemic and lymphatic diseases, as well as skin and musculoskeletal diseases. The drug has been indicated for the treatment of various types of lymphomas, including follicular lymphoma, diffuse large B-cell lymphoma, B-cell lymphoma, large B-cell lymphoma, and mantle-cell lymphoma. It has also shown potential in treating systemic lupus erythematosus, acute lymphoblastic leukemia, B-cell chronic lymphocytic leukemia, and precursor B-cell lymphoblastic leukemia-lymphoma.
Relmacabtagene autoleucel was developed by JW Therapeutics (Shanghai) Co., Ltd., an originator organization based in China. It has received approvals globally. The drug obtained its first approval in China in September 2021, making it a relatively recent addition to the market.
In terms of regulatory status, relmacabtagene autoleucel has undergone priority review, breakthrough therapy designation, and special review project assessments. These regulatory designations highlight the potential significance and urgency of the drug in addressing unmet medical needs.
CAR-T therapies, like relmacabtagene autoleucel, have revolutionized the treatment landscape for certain types of cancers. By genetically modifying a patient's own immune cells to recognize and attack cancer cells, CAR-T therapies offer a personalized and targeted approach to treatment. The approval of relmacabtagene autoleucel signifies a significant advancement in the field of biomedicine, particularly in the treatment of lymphomas and other related diseases.
As an approved CAR-T therapy, relmacabtagene autoleucel holds promise for patients who have not responded to or have relapsed after other treatments. Its approval in China and globally reflects the growing recognition of the therapeutic potential of CAR-T therapies in the medical community. Continued research and development in this field are expected to further expand the applications of CAR-T therapies and improve outcomes for patients with various types of cancers and immune system diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Approved Cell Therapy Medicinal: Axicabtagene Ciloleucel
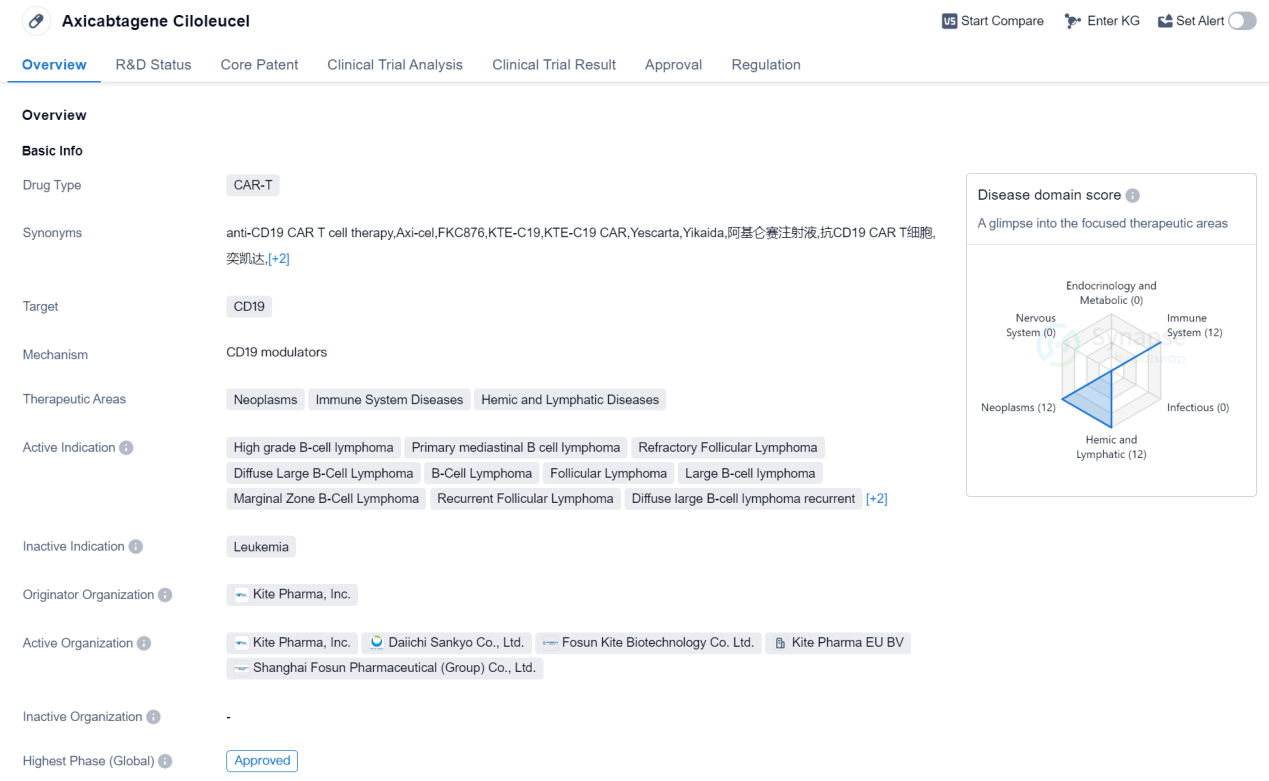
Axicabtagene ciloleucel is a CAR-T therapy drug developed by Kite Pharma, Inc. It falls under the category of CAR-T drugs, which stands for chimeric antigen receptor T-cell therapy. The drug specifically targets CD19, a protein found on the surface of B cells. Axicabtagene ciloleucel is primarily used in the treatment of various neoplasms, immune system diseases, and hemic and lymphatic diseases.
The active indications for this drug include high-grade B-cell lymphoma, primary mediastinal B-cell lymphoma, refractory follicular lymphoma, diffuse large B-cell lymphoma, B-cell lymphoma, follicular lymphoma, large B-cell lymphoma, marginal zone B-cell lymphoma, recurrent follicular lymphoma, diffuse large B-cell lymphoma recurrent, diffuse large B-cell lymphoma refractory, and non-Hodgkin lymphoma.
Axicabtagene ciloleucel received its first approval in the United States in October 2017. The drug has undergone rigorous testing and has reached the highest phase of development, which is the approved phase globally.
In terms of regulatory status, axicabtagene ciloleucel has been granted priority review, breakthrough therapy designation, and orphan drug status. Priority review is a designation given to drugs that have the potential to provide significant improvements in the treatment of serious conditions. Breakthrough therapy designation is granted to drugs that show substantial improvement over existing therapies for life-threatening conditions. Orphan drug status is given to drugs that are intended to treat rare diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In summary, axicabtagene ciloleucel is a CAR-T therapy drug developed by Kite Pharma, Inc. It targets CD19 and is used in the treatment of various neoplasms, immune system diseases, and hemic and lymphatic diseases. The drug has received approvals in the United States and China and has reached the highest phase of development. It has been granted priority review, breakthrough therapy designation, and orphan drug status.