An Overview of UCB's 147 Drug Pipelines ——Top 50 Pharmaceutical Companies R&D Progress
UCB SA is a pharmaceutical organization that was founded in 1928 and is located in Brussels Hoofdstedelijk Gewest, Belgium. The company operates in the field of biomedicine and focuses on the development of drugs for various therapeutic areas.
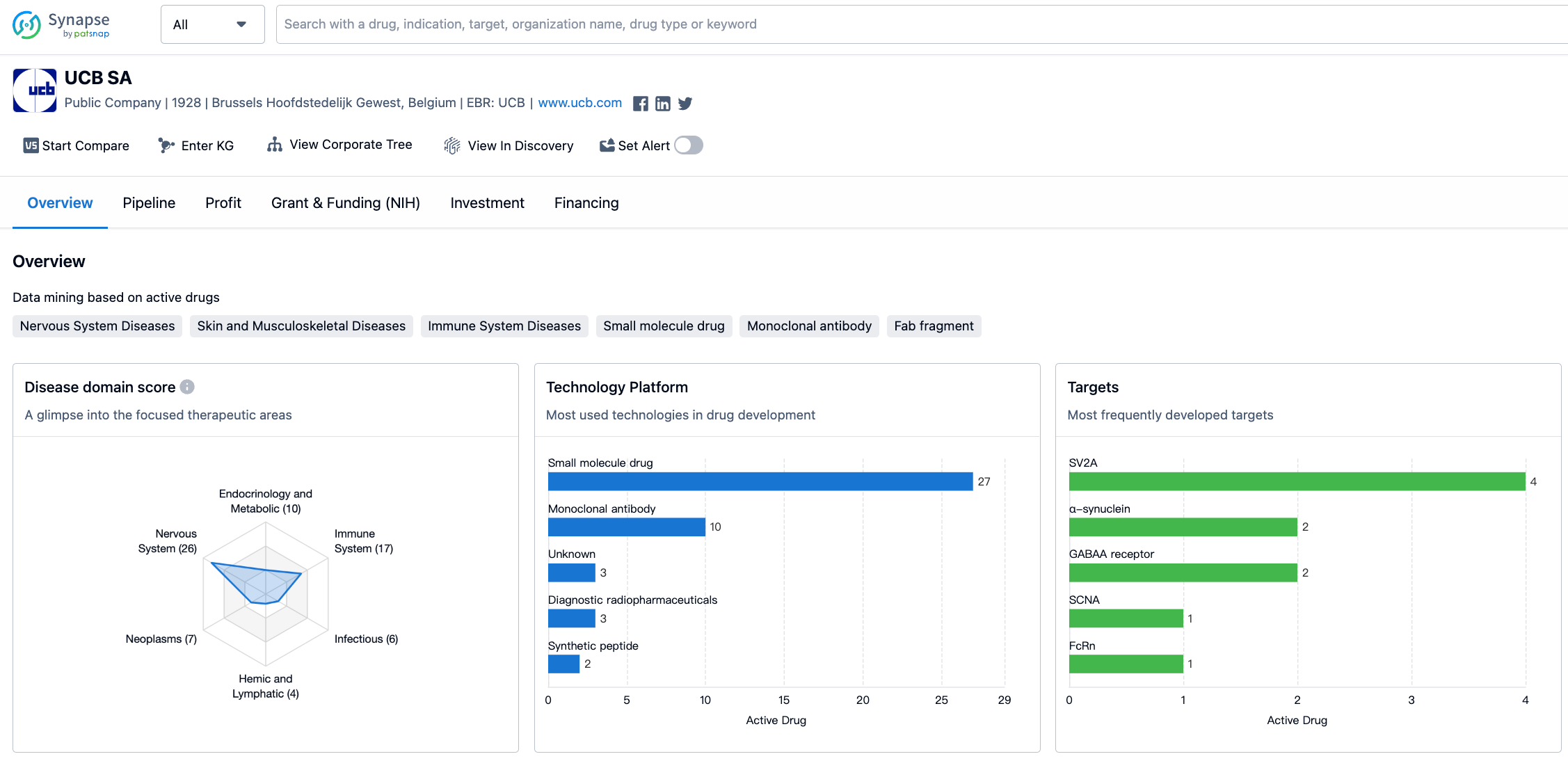
The organization has developed drugs for a wide range of therapeutic areas, with the highest drug count in Nervous System Diseases, followed by Immune System Diseases and Skin and Musculoskeletal Diseases. This indicates that UCB SA has a strong focus on developing drugs for these areas. Other therapeutic areas with significant drug counts include Endocrinology and Metabolic Disease, Other Diseases, and Congenital Disorders. On the other hand, therapeutic areas such as Cardiovascular Diseases and Urogenital Diseases have a relatively lower drug count, suggesting that UCB SA may have a lesser focus on these areas.
The target SV2A has the highest drug count, indicating that UCB SA has invested significant resources in developing drugs targeting this specific protein. Other targets with multiple drug developments include α-synuclein and GABAA receptor. This suggests that UCB SA has a strong focus on developing drugs that interact with these targets. Additionally, there are several targets with a single drug count, indicating that UCB SA has explored a diverse range of targets in its drug development efforts.
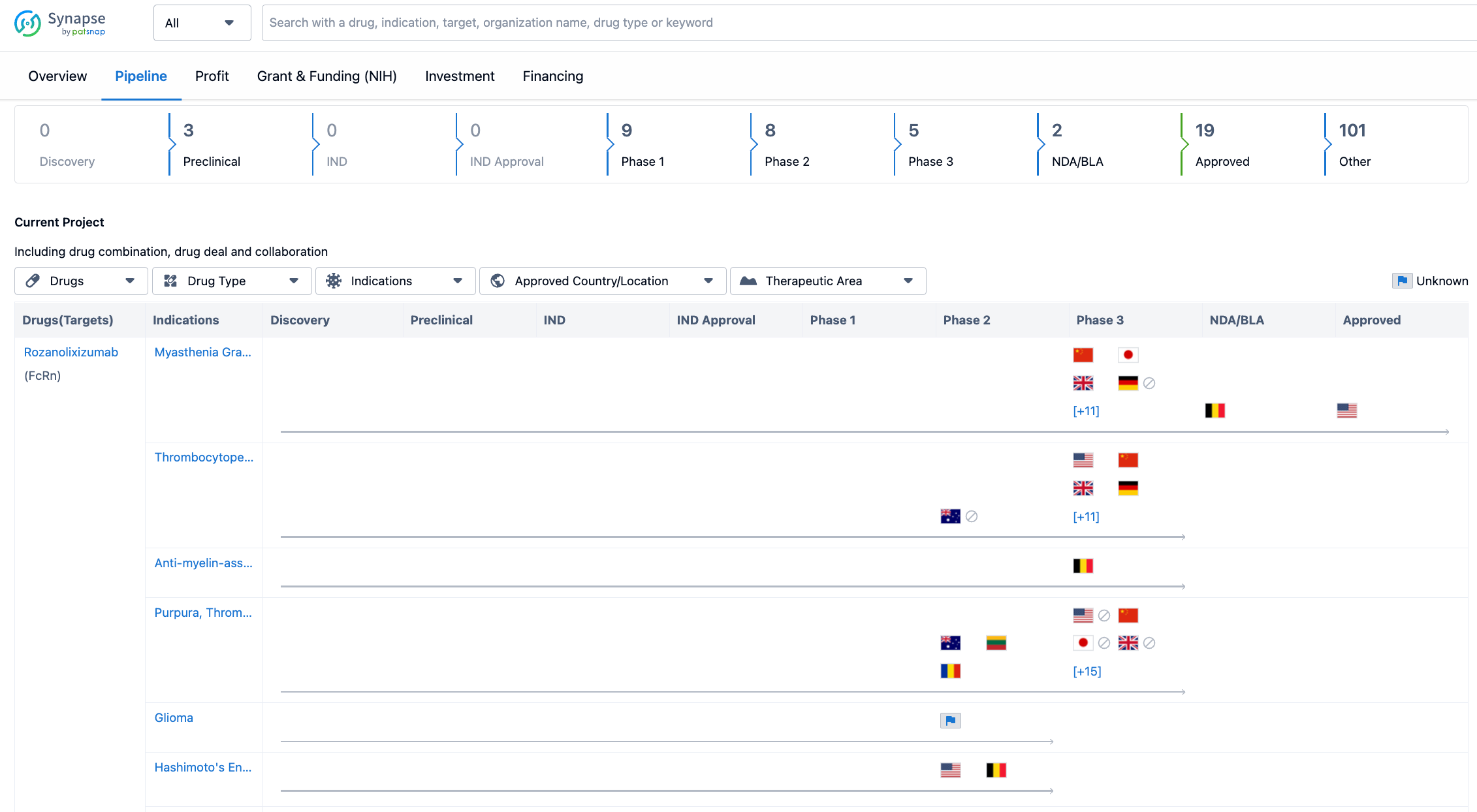
The organization has three drugs in the preclinical stage, indicating that they are in the early stages of development. There are no drugs in the discovery stage, suggesting that UCB SA may have already identified potential drug candidates for further development. In terms of clinical trials, UCB SA has a significant number of drugs in Phase 1, Phase 2, and Phase 3, indicating that they are actively conducting clinical trials to evaluate the safety and efficacy of their drug candidates. The organization has two drugs in the NDA/BLA stage. Furthermore, UCB SA has 19 drugs that have been approved, indicating that they have successfully brought several drugs to the market. Lastly, there are 101 drugs categorized as "Other," which could include drugs in various stages of development or those that do not fit into the traditional drug development phases.
In summary, UCB SA is a pharmaceutical organization that has been operating since 1928 and is based in Brussels Hoofdstedelijk Gewest, Belgium. The company focuses on the development of drugs for various therapeutic areas, with a strong emphasis on Nervous System Diseases, Immune System Diseases, and Skin and Musculoskeletal Diseases. They have developed drugs targeting a wide range of proteins, with SV2A being the most frequently targeted. UCB SA has an active pipeline with drugs in different stages of development, including preclinical, clinical trials, and approved drugs. This indicates that the organization is committed to advancing its drug candidates through the various stages of development and bringing them to the market.