Ritlecitinib - Dual Inhibitor of JAK3/TEC
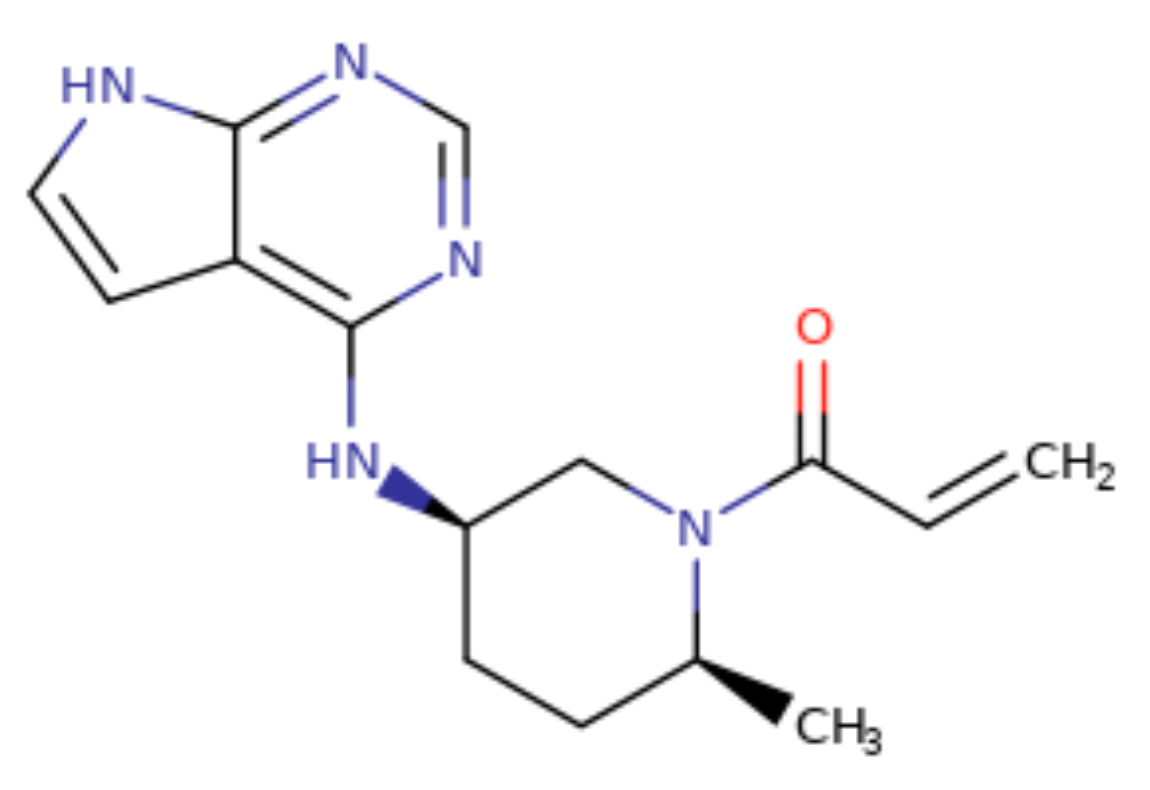
Ritlecitinib, trade name LITFULO, is a small molecule drug targeting JAK3 and TEC (see Figure 2-9 for molecular structure). This drug is a once-daily oral treatment, developed by Pfizer Inc. On June 23, 2023, the Type 1 new drug application submitted has been approved by the FDA for treatment of individuals 12 years of age and older with severe alopecia areata.
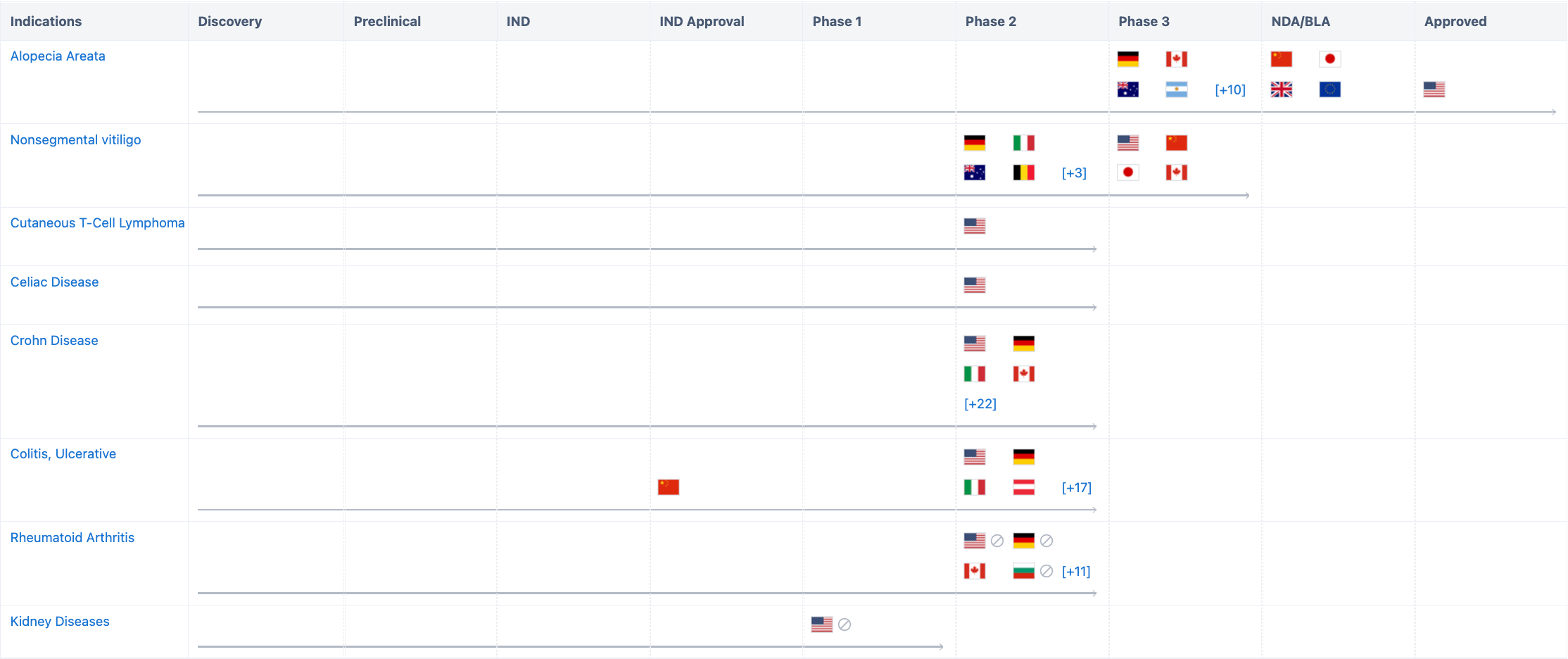
According to “Synapse”, the drug involves a total of 8 indications, including Alopecia Areata, Nonsegmental vitiligo, Cutaneous T-Cell Lymphoma, Celiac Disease, Crohn Disease, Colitis, Ulcerative, Rheumatoid and Kidney Diseases. The Phase I clinical trial on renal insufficiency was initiated in August 2019, but was terminated due to extensive delays in COVID-19. These indications have all entered the clinical trial stage. The research progress on alopecia areata has not only been approved in the United States, but also has submitted listing applications in China, Europe, Japan, and the UK. For more detailed information on R&D status, core patents, analysis, etc, please click on the image link below.
Mechanism of Action
It is the first and only treatment approved by the FDA for adolescents (12+) with severe alopecia areata.Ritlecitinib irreversibly inhibits Janus kinase 3 (JAK3) and the tyrosine kinase expressed in hepatocellular carcinoma (TEC) kinase family by blocking the adenosine triphosphate (ATP) binding site. In cellular settings, ritlecitinib inhibits cytokine induced STAT phosphorylation mediated by JAK3-dependent receptors. Additionally, ritlecitinib inhibits signaling of immune receptors dependent on TEC kinase family members. The relevance of inhibition of specific JAK or TEC family enzymes to therapeutic effectiveness is not currently known.
Efficacy and safety
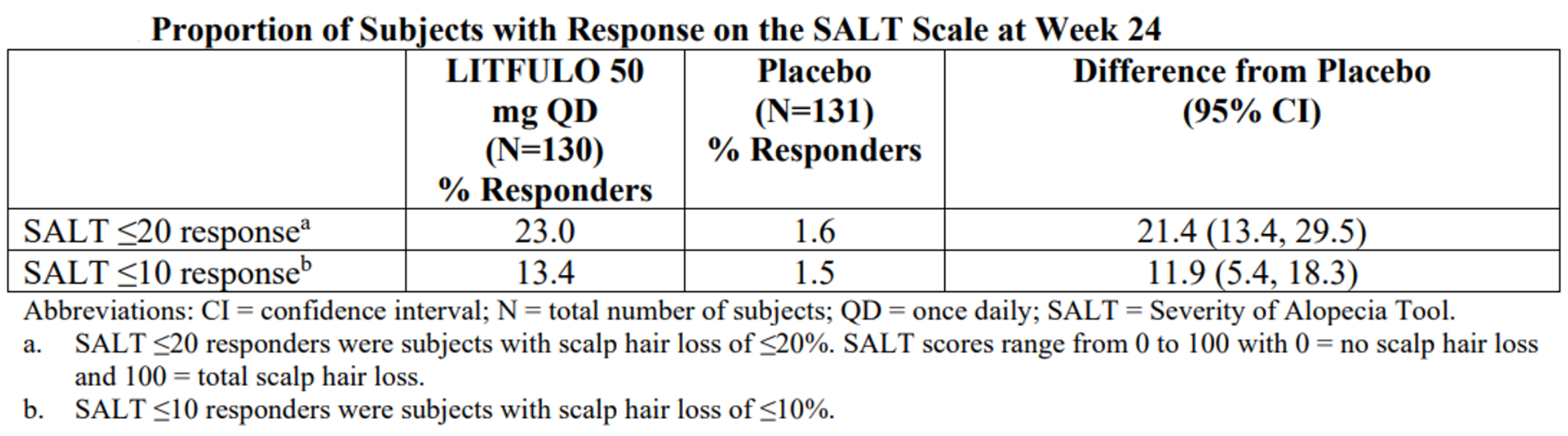
The FDA approval was based on results(Figure 2-10) of clinical trials in alopecia areata. The ALLEGRO Phase 2b/3 trial (NCT03732807) , which enrolled 718 patients with 50% or more scalp hair loss as measured by the Severity of Alopecia Tool (SALT), evaluated the efficacy and safety of LITFULO at 118 sites in 18 countries. In this pivotal study, 23% of patients treated with LITFULO 50 mg had 80% or more scalp hair coverage (SALT≤20) after six months compared to 1.6% with placebo. The efficacy and safety of LITFULO were consistent between adolescents (12 through 17 years of age) and adults (18 years of age and older).
The most common adverse events (AEs) reported in at least 4% of patients with LITFULO include headache (10.8%), diarrhea (10%), acne (6.2%), rash (5.4%), and urticaria (4.6%).
Competitive Landscape
According to “Synapse”, at present, this drug is the only one that targets both JAK3 and TEC simultaneously.