Analysis on the Clinical Research Progress of CTLA-4 Inhibitors
Cytotoxic T Lymphocyte-Associated Antigen-4 (CTLA-4), also known as CD152, is a transmembrane protein on the surface of T cells encoded by the CTLA-4 gene. Its gene is located at band 3, region 3 of the long arm of chromosome 2 (2q33). It is mainly expressed on the surface of activated T lymphocytes and has a close relationship with the cooperation stimulating molecule receptor (CD28) on the surface of T cells in terms of gene structure, chromosomal localization, sequence homology, and gene expression. It can bind with the cooperating stimulating molecule (B7) on the surface of antigen-presenting cells (APC). However, unlike CD28, CTLA-4 inhibits T cell activation after binding with the B7 molecule. This is done through its extracellular domain competing with the ligand, occupying the B7 ligand of APC, thereby blocking the CD28 signal; or through its intracellular domain mediating a negative signal to inhibit T cell activation.
Exogenous antigens and tumour-specific related target antigens stimulate the body's anti-tumour immunity. In the initial stage, due to the low level of CTLA-4, the B7 and CD28 actions dominate, activating T cells to secrete cytokines such as IL-2 and proliferate and differentiate into effector T cells. As the expression of CTLA-4 in activated T cells increases, in the later stage of immune response CTLA-4 competitively binds with the B7 molecule with CD28, inhibiting T cells from moving from G phase to S phase and the activity of IL-2 transcription factor, thereby down-regulating or terminating T cell response.
Immune responses to tumor cells start with the exposure and release of tumor-specific antigens, followed by antigen-presenting cells (APC) engulfing tumor antigens and presenting them to T cells, forming sensitized and activated effector T cells in lymph nodes. Afterwards, T cells infiltrate the tumor under the action of chemotactic factors and release more tumor antigens.
CTLA-4 exists as a homodimer, consisting of an extracellular region, a transmembrane region and a cytoplasmic region, mainly expressed on activated CD4+ and CD8+ T cells, also associated with Treg cells surface. CTLA-4 expressed on Treg surface has an important function in driving Treg, gathering in tumor tissue to suppress anti-tumor immunity. Blocking CTLA-4 dulls the function of tumor-infiltrating Treg, thus enhancing intra-tumoral immune response. Therefore, CTLA-4 is considered an immune molecule that inhibits the body's anti-tumor response and is a hotspot and direction for current tumor-targeted immunotherapy.
CTLA-4 Competitive Landscape
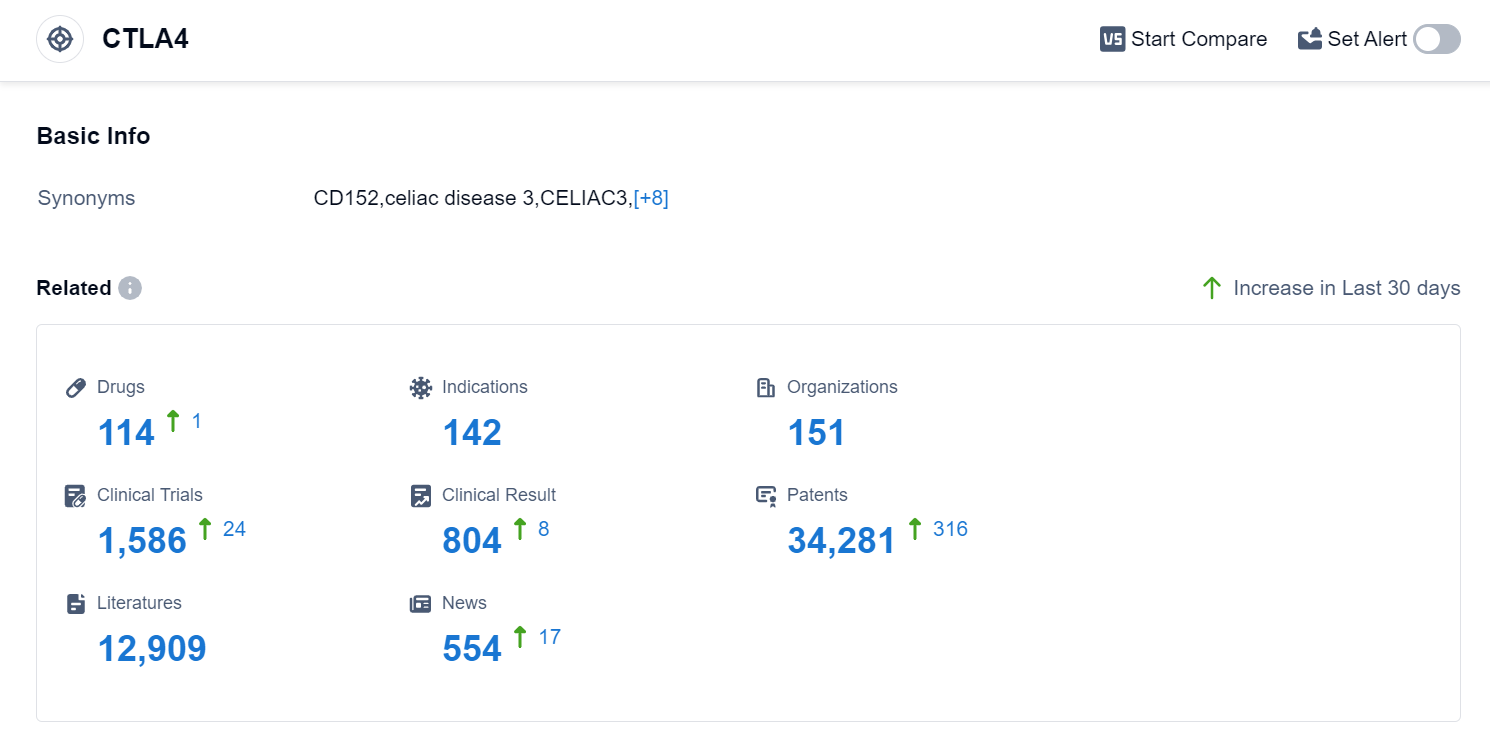
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 18 Sep 2023, there are a total of 114 CTLA4 drugs worldwide, from 151 organizations, covering 142 indications, and conducting 1586 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
The analysis of the target CTLA-4 reveals a competitive landscape with multiple companies actively involved in the development of drugs. AstraZeneca PLC, Bristol Myers Squibb Co., and Akeso, Inc. are among the companies growing the fastest under this target. The approved drugs under the target CTLA-4 have indications in various types of cancer, including non-small cell lung cancer, hepatocellular carcinoma, melanoma, and colorectal cancer.
Monoclonal antibodies, bispecific antibodies, and biosimilars are the drug types progressing most rapidly under the target CTLA-4, indicating intense competition. China, the United States, and the European Union are the leading countries/locations in the development of drugs targeting CTLA-4.
Overall, the target CTLA-4 presents a promising area for the development of innovative drugs in the pharmaceutical industry, with a focus on cancer indications. The competition among companies and the diversity of drug types highlights the potential for advancements in the treatment of various cancers.
The globally approved CTLA-4 inhibitor on the market: Ipilimumab
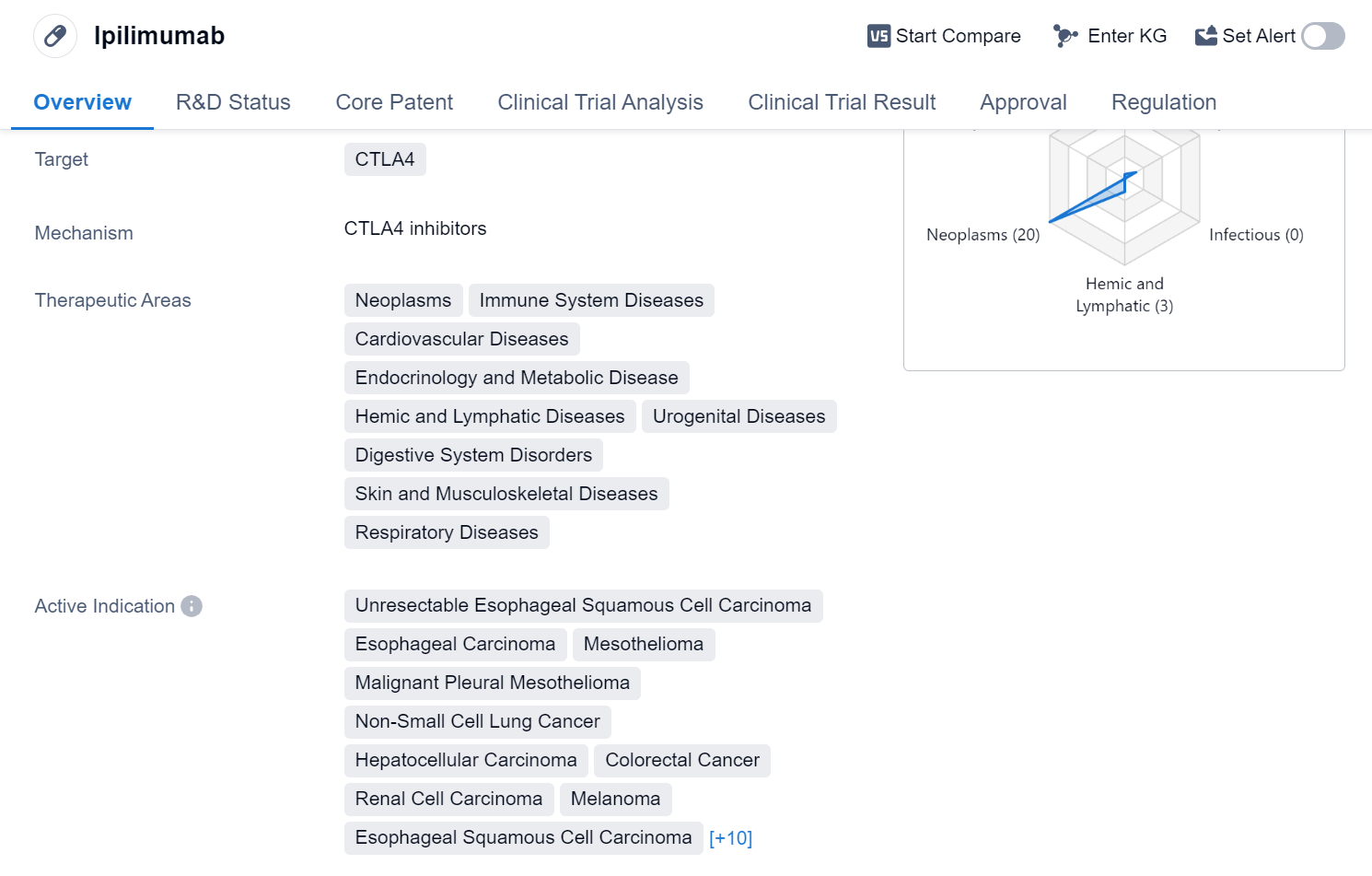
Ipilimumab is a monoclonal antibody drug that targets CTLA-4, a protein found on the surface of certain immune cells. It has been approved for use in various therapeutic areas, including neoplasms, immune system diseases, cardiovascular diseases, endocrinology and metabolic diseases, hemic and lymphatic diseases, urogenital diseases, digestive system disorders, skin and musculoskeletal diseases, and respiratory diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug has shown efficacy in treating a wide range of cancers and has been approved for multiple indications. These include unresectable esophageal squamous cell carcinoma, esophageal carcinoma, mesothelioma, malignant pleural mesothelioma, non-small cell lung cancer, hepatocellular carcinoma, colorectal cancer, renal cell carcinoma, melanoma, bladder cancer, transitional cell carcinoma, gastroesophageal junction cancer, stomach cancer, metastatic hormone refractory prostate cancer, ovarian cancer, Hodgkin's lymphoma, multiple myeloma, non-Hodgkin lymphoma, and solid tumors.
Ipilimumab was developed by Bristol Myers Squibb Co., a renowned pharmaceutical company. It received its first approval in the United States in March 2011 and has since gained approval in other countries as well. The drug has undergone rigorous regulatory processes and has been granted accelerated approval, fast track designation, orphan drug status, and breakthrough therapy designation.
The highest phase of development for Ipilimumab is approved. This achievement highlights the drug's potential to address unmet medical needs and provide significant benefits to patients.
Ipilimumab's approval in China further expands its reach and potential impact on patients in the country. This signifies the drug's global recognition and acceptance as a valuable treatment option.
In summary, Ipilimumab is a monoclonal antibody drug developed by Bristol Myers Squibb Co. It targets CTLA-4 and has been approved for various therapeutic areas, particularly in the treatment of different types of cancers. The drug has undergone extensive regulatory processes and has received accelerated approval, fast track designation, orphan drug status, and breakthrough therapy designation. Its approval in both the United States and China demonstrates its global significance and potential to improve patient outcomes.
Tremelimumab
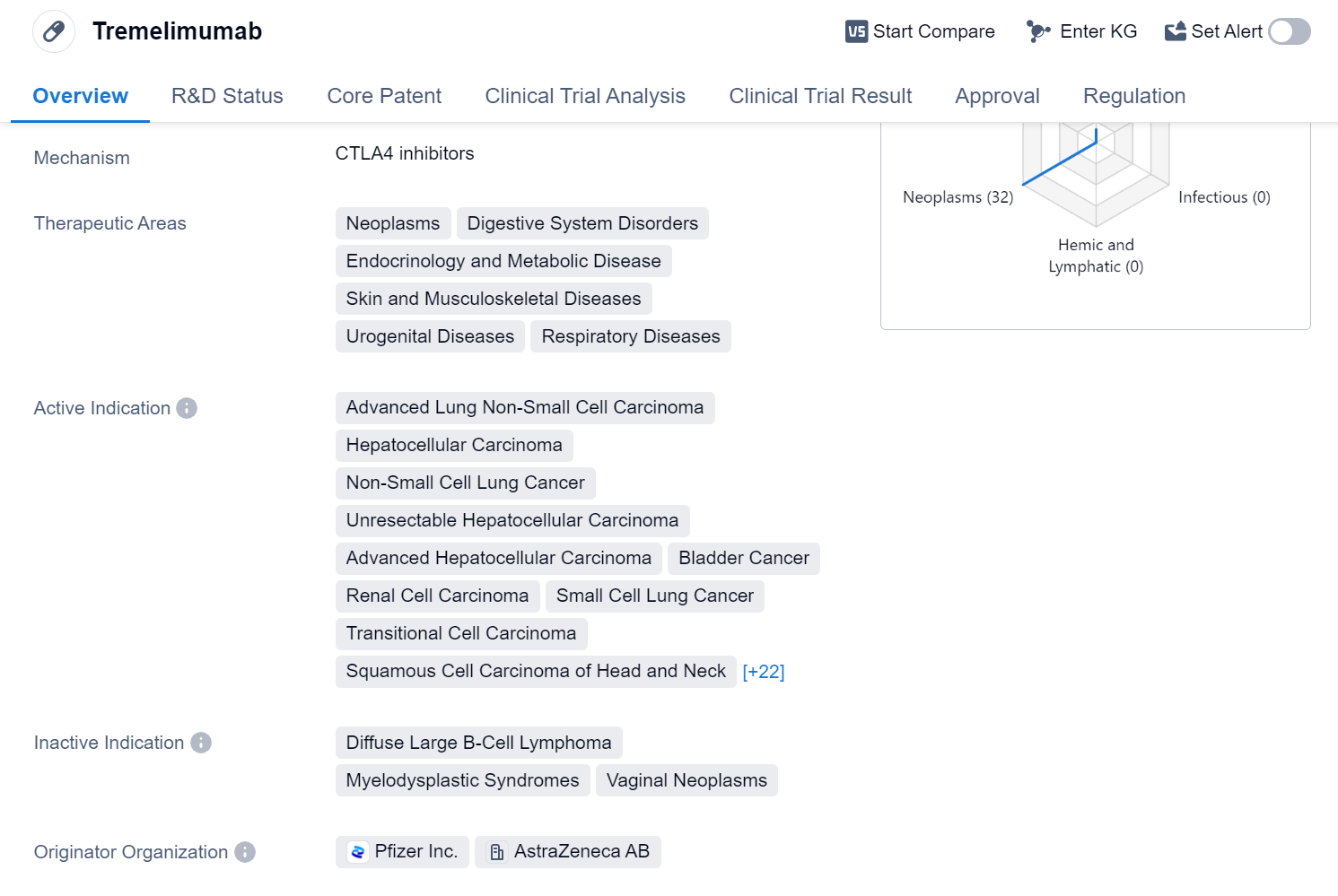
Tremelimumab is a monoclonal antibody drug that targets CTLA-4, a protein involved in regulating the immune system. It has shown potential in treating various neoplasms and diseases related to the digestive system, endocrinology and metabolism, skin and musculoskeletal system, urogenital system, and respiratory system.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug has been indicated for the treatment of several advanced cancers, including lung non-small cell carcinoma, hepatocellular carcinoma, bladder cancer, renal cell carcinoma, and pancreatic cancer, among others. It has also shown promise in treating head and neck squamous cell carcinoma, mesothelioma, uterine cervical cancer, and thyroid cancer.
Tremelimumab was developed by Pfizer Inc. and AstraZeneca AB, and it has reached the highest phase of development, which is approved globally. It received its first approval in the United States in October 2022. The drug has also entered phase 3 of development in China.
In terms of regulatory status, Tremelimumab has been designated as an orphan drug, indicating its potential to treat rare diseases or conditions. It has also undergone priority review, which expedites the regulatory process for drugs that address significant unmet medical needs.
Overall, Tremelimumab represents a significant advancement in the field of biomedicine, particularly in the treatment of various cancers. Its monoclonal antibody nature and targeting of CTLA-4 make it a promising therapeutic option for patients with advanced cancers who have limited treatment options. The drug's approval in the United States and ongoing development in China further highlight its potential global impact. With its orphan drug designation and priority review status, Tremelimumab demonstrates its potential to address unmet medical needs and provide new treatment options for patients.