Analysis on the Clinical Research Progress of IL-23 inhibitors
IL-23, or Interleukin-23, is a crucial cytokine that plays a significant role in the human body's immune response. It is primarily produced by activated antigen-presenting cells, such as dendritic cells and macrophages. IL-23 acts as a mediator between innate and adaptive immunity by stimulating the production of pro-inflammatory cytokines and promoting the differentiation and expansion of T-helper 17 (Th17) cells. Th17 cells, in turn, produce various cytokines that contribute to inflammation and immune responses against pathogens. However, dysregulation of IL-23 signaling has been associated with several autoimmune diseases, making it an important target for therapeutic interventions in the pharmaceutical industry.
IL-23 Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 20 Sep 2023, there are a total of 39 IL-23 drugs worldwide, from 68 organizations, covering 31 indications, and conducting 279 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.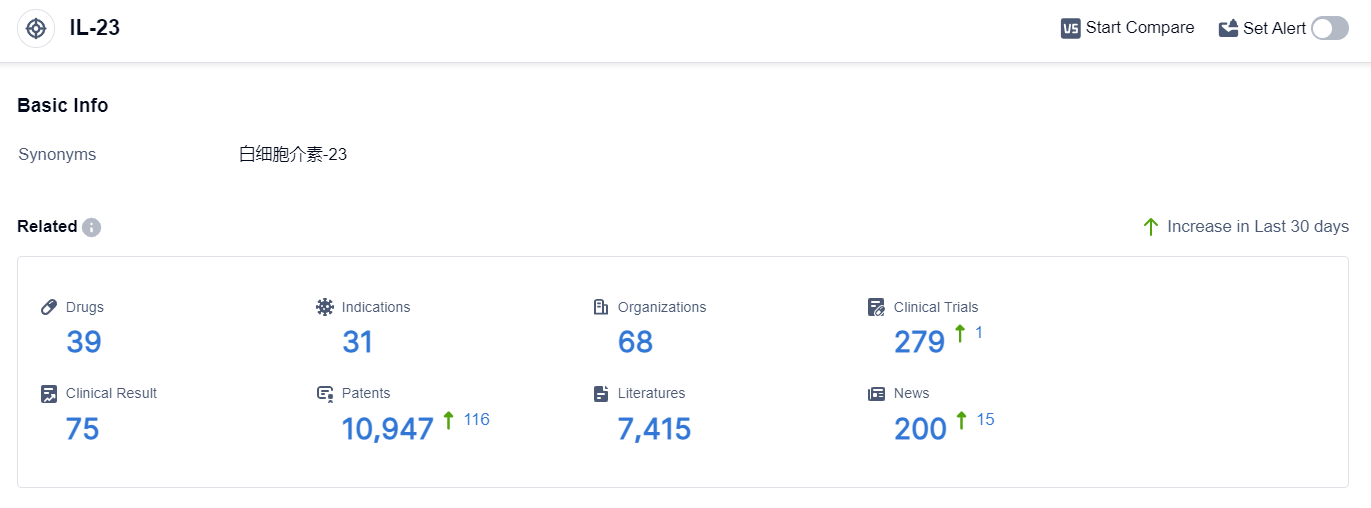
The analysis of target IL-23 reveals a competitive landscape with multiple companies actively involved in research and development. Johnson & Johnson, Alvotech Swiss AG, and AstraZeneca PLC are among the companies showing significant progress.
Approved indications for IL-23 drugs include psoriasis, Crohn Disease, plaque psoriasis, colitis, ulcerative, and arthritis, psoriatic. Monoclonal antibodies and biosimilars are the most rapidly progressing drug types, indicating intense competition.
The United States, European Union, and China are the leading countries in IL-23 drug development, with China showing notable progress. Overall, the target IL-23 presents a promising opportunity for the pharmaceutical industry, with a wide range of indications and potential applications.
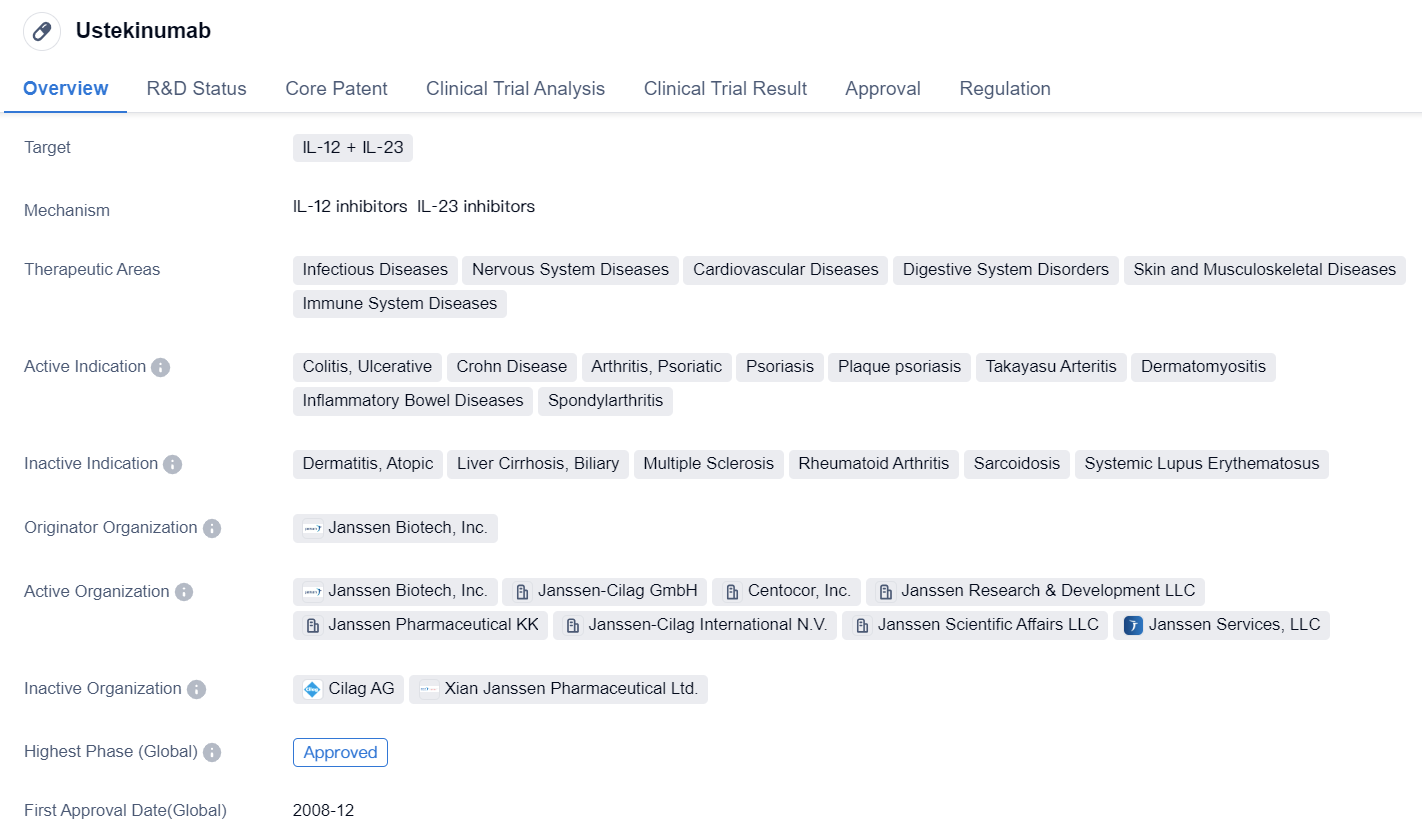
IL-23 inhibitors approved for marketing: Ustekinumab
Ustekinumabis a monoclonal antibody drug that targets IL-12 and IL-23. It has been approved for use in various therapeutic areas, including infectious diseases, nervous system diseases, cardiovascular diseases, digestive system disorders, skin and musculoskeletal diseases, and immune system diseases. The drug is indicated for the treatment of colitis, ulcerative, Crohn disease, arthritis, psoriatic, psoriasis, plaque psoriasis, Takayasu arteritis, dermatomyositis, inflammatory bowel diseases, and spondylarthritis.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The originator organization of Ustekinumab is Janssen Biotech, Inc. It received its first approval in Canada in December 2008. The drug has also been approved in other countries globally. In China, it has received approval as well.
Ustekinumab falls under the category of overseas new drugs urgently needed in clinical settings and is also classified as an orphan drug. This indicates that the drug addresses a significant unmet medical need and is intended for the treatment of rare diseases or conditions.
As a monoclonal antibody, Ustekinumab works by targeting specific proteins in the immune system, namely IL-12 and IL-23. By inhibiting these proteins, the drug helps to regulate the immune response and reduce inflammation associated with various diseases.
The approval of Ustekinumab in multiple therapeutic areas highlights its versatility and potential to address a wide range of medical conditions. Its efficacy and safety profile have been evaluated through clinical trials and regulatory processes, leading to its approval for use in patients.
In summary, Ustekinumab is a monoclonal antibody drug that targets IL-12 and IL-23. It has been approved for the treatment of various diseases in different therapeutic areas. The drug was first approved in Canada in 2008 and has since received approval in other countries globally, including China. Its classification as an overseas new drug urgently needed in clinical settings and an orphan drug underscores its importance in addressing unmet medical needs.
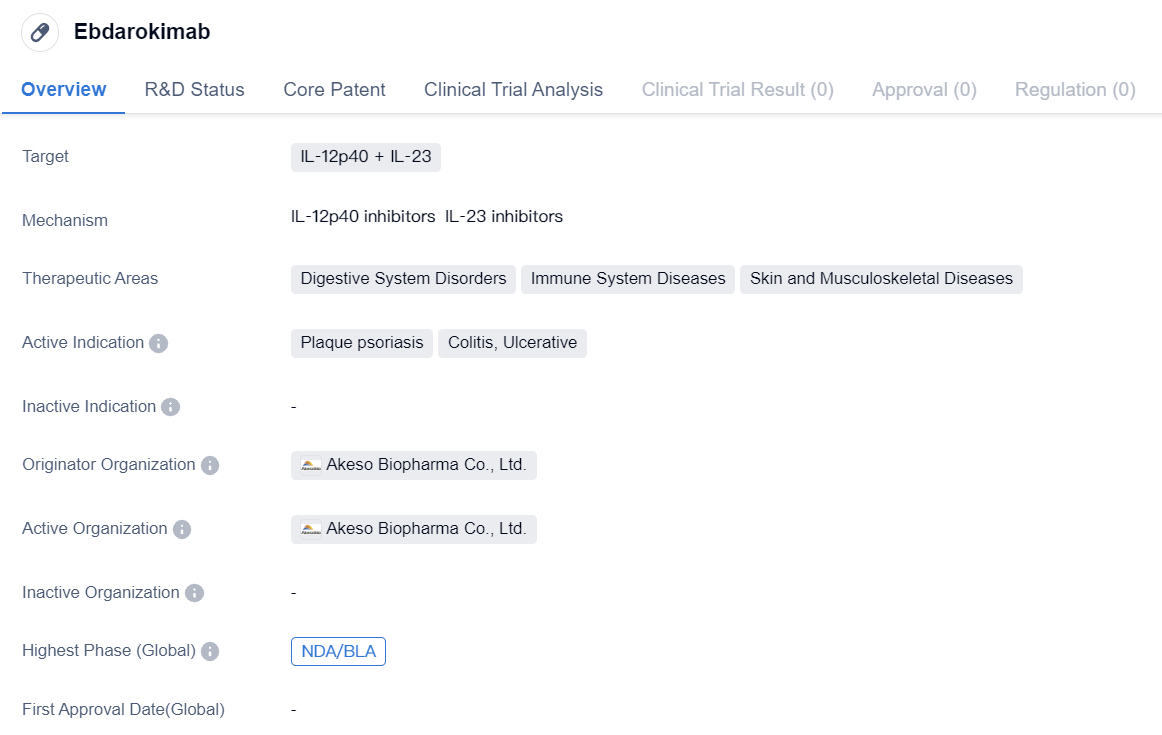
Ebdarokimab
Ebdarokimab is a monoclonal antibody drug that targets IL-12p40 and IL-23. It is being developed by Akeso Biopharma Co., Ltd., a pharmaceutical company specializing in biomedicine. The drug is primarily intended for the treatment of various disorders related to the digestive system, immune system, skin, and musculoskeletal system.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The therapeutic areas that Ebdarokimab focuses on include digestive system disorders, immune system diseases, and skin and musculoskeletal diseases. This suggests that the drug may have potential applications in treating conditions such as plaque psoriasis, colitis, and ulcerative colitis. These diseases are characterized by inflammation and immune system dysregulation, and targeting IL-12p40 and IL-23 may help to alleviate symptoms and improve patient outcomes.
Ebdarokimab has reached the NDA/BLA stage, both globally and in China. NDA stands for New Drug Application, while BLA stands for Biologics License Application. These phases indicate that the drug has undergone extensive preclinical and clinical testing and is now being submitted for regulatory approval. This suggests that Ebdarokimab has shown promising results in early studies and may have the potential to become a commercially available treatment option in the near future.
Based on the available information, it can be concluded that Ebdarokimab is a monoclonal antibody drug targeting IL-12p40 and IL-23, with potential applications in the treatment of various disorders related to the digestive system, immune system, skin, and musculoskeletal system. The drug has reached the NDA/BLA stage, indicating its potential for regulatory approval and commercialization. However, further research and clinical trials are likely needed to fully evaluate the safety and efficacy of Ebdarokimab in treating specific indications such as plaque psoriasis, colitis, and ulcerative colitis.