Analysis on the Clinical Research Progress of Myostatin inhibitors
Myostatin, a member of the transforming growth factor beta (TGF‐β) superfamily that is highly expressed in skeletalmuscle, was first described in 1997. It has been known that loss of myostatin function induces an increase in musclemass in mice, cow, dogs and humans. Therefore, myostatin and its receptor have emerged as a therapeutic target forloss of skeletal muscle such as sarcopenia and cachexia, as well as muscular dystrophies. At the molecular level,myostatin binds to and activates the activin receptor IIB (ActRIIB)/Alk 4/5 complex. Therapeutic approaches thereforeare being taken both pre‐clinically and clinically to inhibit the myostatin signaling pathway. Several myostatininhibitors , including myostatin antibodies, anti‐myostatin peptibody, activin A antibody, soluble (decoy) forms ofActRIIB (ActRⅡB‐Fc), anti‐myostatin adnectin, ActRⅡB antibody have been tested in the last decade.
Myostatin is a protein that plays a crucial role in regulating muscle growth and development in the human body. It acts as a negative regulator, inhibiting muscle cell proliferation and differentiation. By binding to specific receptors on muscle cells, myostatin limits their ability to grow and increase in size. This protein is essential for maintaining muscle homeostasis and preventing excessive muscle growth. However, mutations or deficiencies in myostatin can lead to increased muscle mass and strength, as seen in certain genetic conditions. Understanding the role of myostatin has significant implications for developing therapies to treat muscle wasting diseases and enhancing athletic performance.
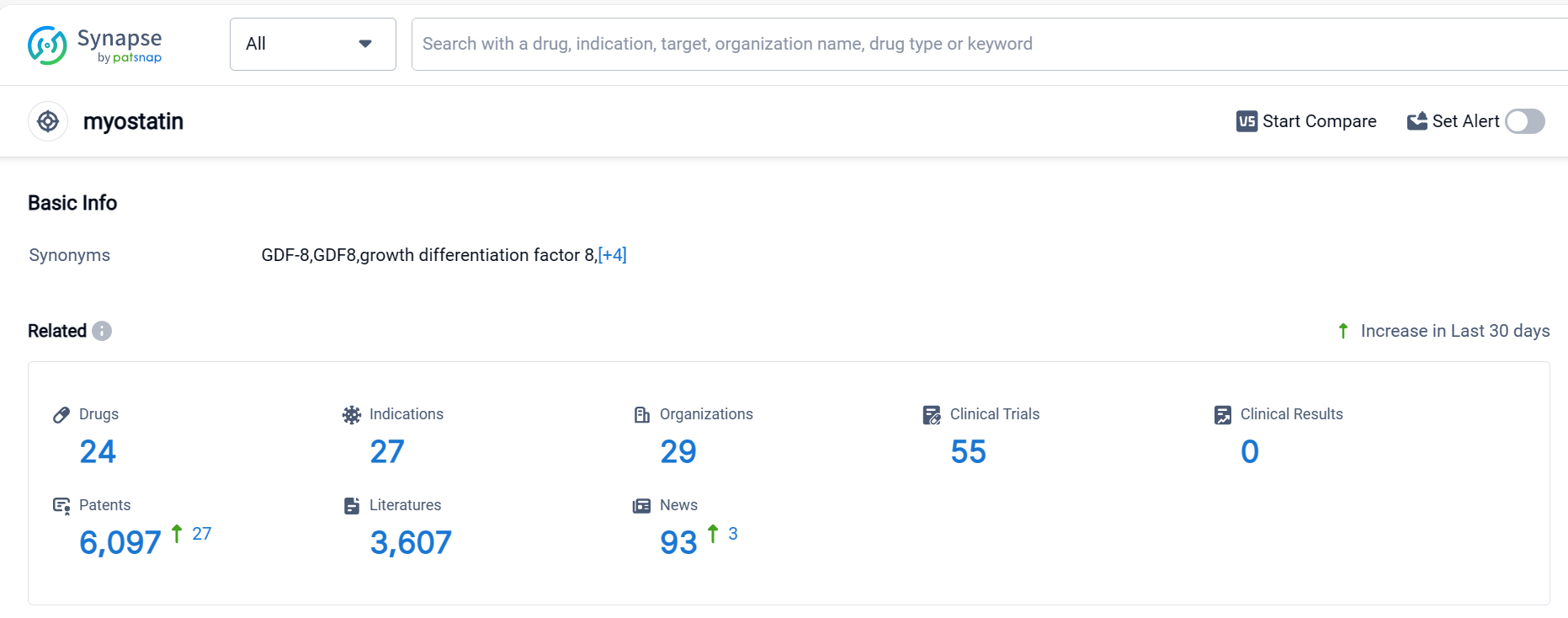
MYOSTATIN Competitive Landscape
According to Patsnap Synapse, as of 1 Oct 2023, there are a total of 31 MR drugs worldwide, from 51 organizations, covering 56 indications, and conducting 870 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
Based on the analysis of the provided data, the current competitive landscape of the target MR is characterized by the involvement of multiple companies, with Bayer AG and Pfizer Inc. leading in terms of drug approvals. The most common approved indications include hypertension, contraception, and heart failure. Small molecule drugs dominate the development phases, indicating their effectiveness and market potential. The United States, European Union, Japan, and China are the key countries driving development under the target MR.
To gain a more comprehensive understanding of the competitive landscape and future development, further analysis beyond the provided data is recommended. This may include examining additional companies, indications, drug types, and countries/locations involved in the target MR. Additionally, monitoring the progress of biosimilars and their impact on the innovative drug market is crucial. Overall, the target MR presents opportunities for growth and innovation in the pharmaceutical industry.
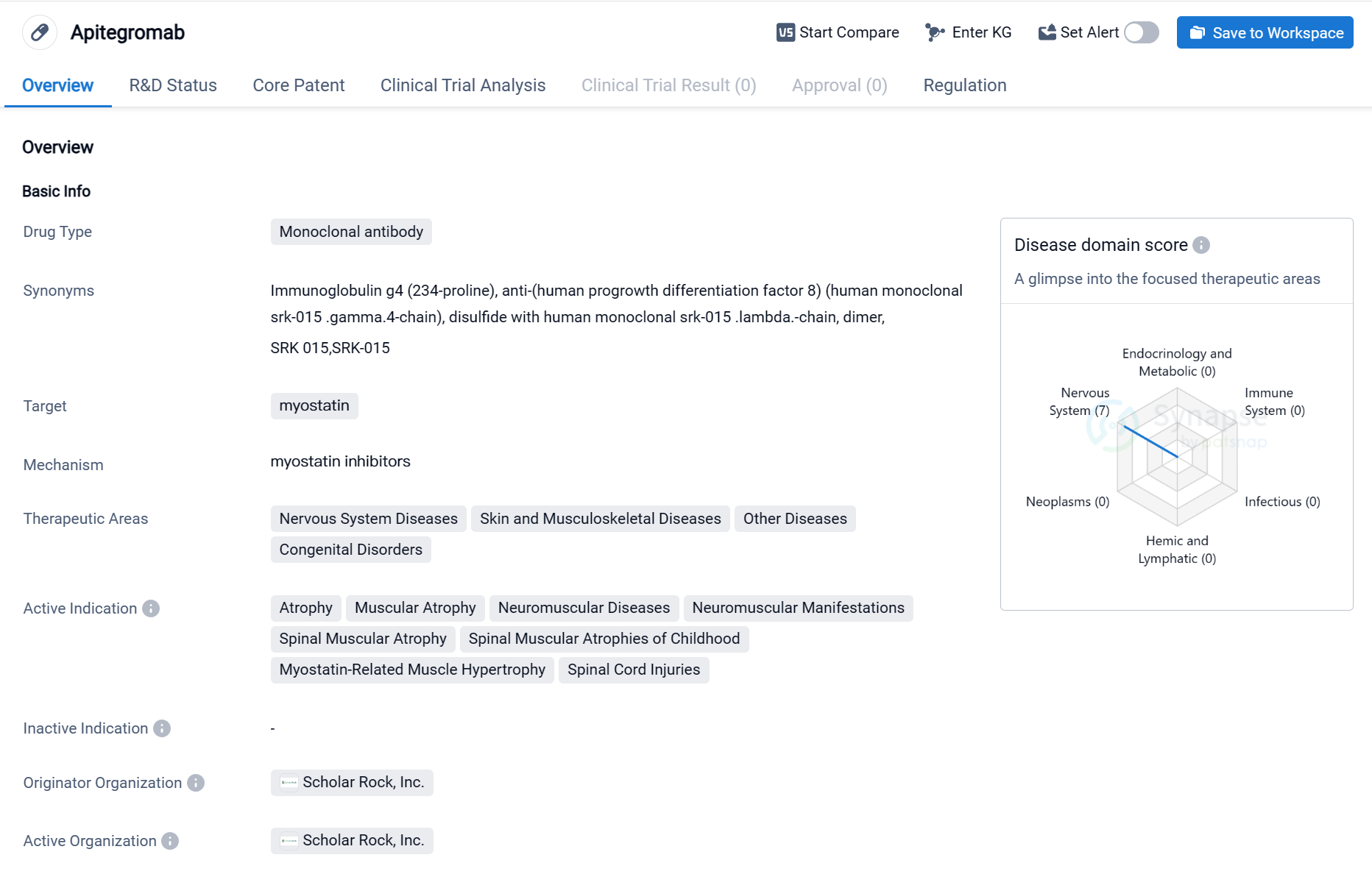
Selective Myostatin Inhibitors:Apitegromab
Apitegromab is a monoclonal antibody drug developed by Scholar Rock, Inc. It falls under the therapeutic area of biomedicine and specifically targets myostatin. Myostatin is a protein that regulates muscle growth, and inhibiting its activity can potentially have therapeutic benefits in various diseases.
The drug has shown promise in treating a range of conditions related to the nervous system, skin and musculoskeletal system, as well as other diseases and congenital disorders. Some of the active indications for Apitegromab include atrophy, muscular atrophy, neuromuscular diseases, neuromuscular manifestations, spinal muscular atrophy, spinal muscular atrophies of childhood, myostatin-related muscle hypertrophy, and spinal cord injuries.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Apitegromab is currently in the highest phase of clinical development, which is Phase 3. This indicates that the drug has already undergone extensive testing in preclinical and early clinical stages and is now being evaluated in a larger population to assess its safety and efficacy.
In terms of regulatory status, Apitegromab has been granted several designations that provide incentives and expedited pathways for its development. These include Orphan Drug designation, which is granted to drugs intended to treat rare diseases, PRIME (PRIority MEdicines) designation, which is given to drugs with the potential to address unmet medical needs, Fast Track designation, which expedites the development and review of drugs for serious conditions, and Rare Pediatric Disease designation, which provides incentives for the development of drugs for pediatric rare diseases.
Overall, Apitegromab shows promise as a potential treatment for various diseases and disorders related to muscle atrophy and neuromuscular conditions. Its advancement to Phase 3 clinical trials and the regulatory designations it has received highlight the potential significance of this drug in addressing unmet medical needs in these therapeutic areas. Further research and clinical trials will be necessary to fully evaluate the safety and efficacy of Apitegromab and determine its potential impact on patient outcomes.