UCB's innovative therapy, IL-17A/F inhibitor bimekizumab, has been approved by the FDA for the treatment of plaque psoriasis
Recently, UCB announced that the FDA has approved Bimzelx(bimekizumab) for the treatment of moderate to severe plaque psoriasis in adult patients who are candidates for systemic treatment or phototherapy. Bimekizumab is the world's first IL-17A/F inhibitor approved for the treatment of moderate to severe plaque psoriasis.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
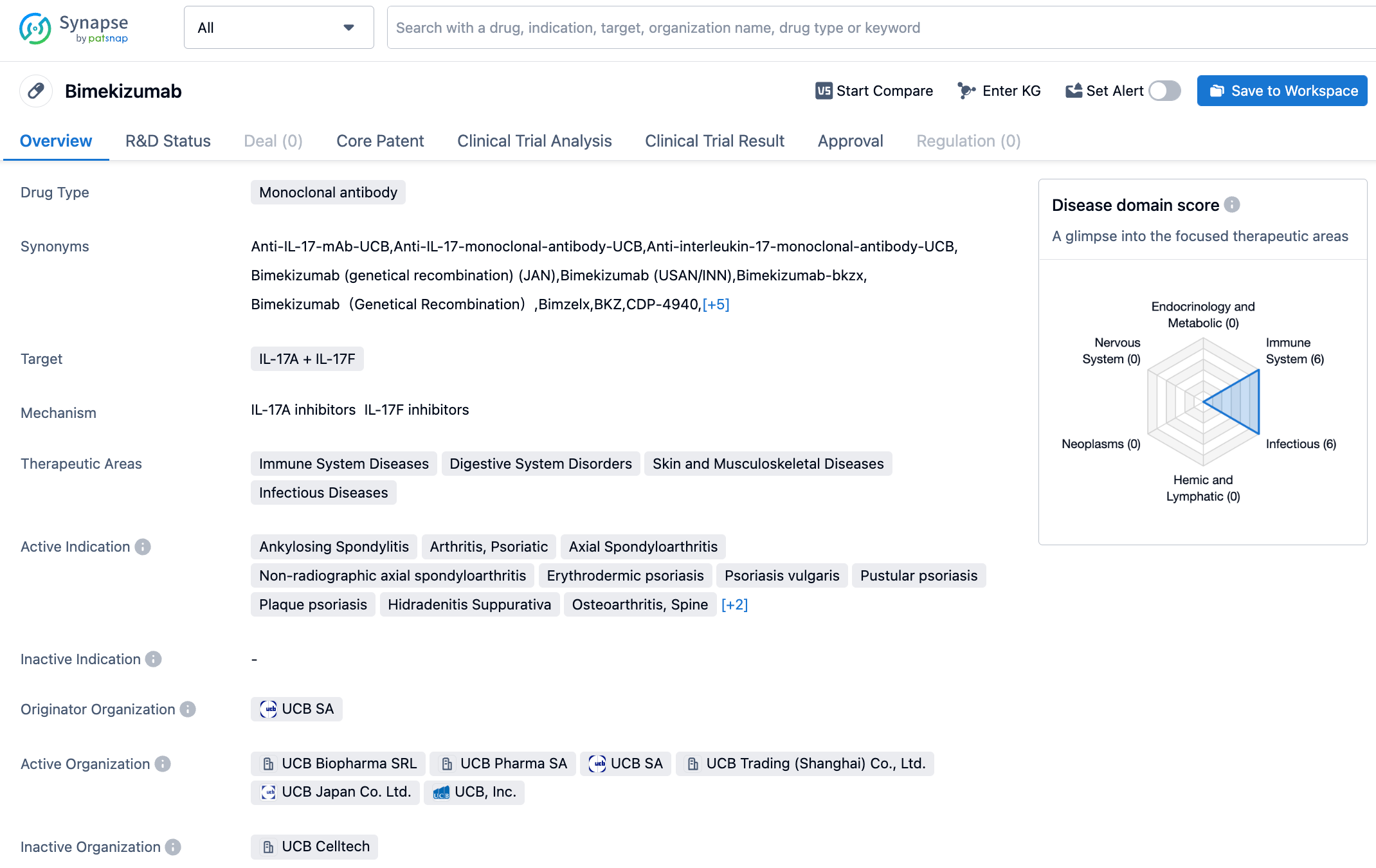
Bimekizumab is a humanized monoclonal IgG1 antibody, designed to simultaneously inhibit two key cytokines driving the inflammatory process, Interleukin 17A(IL-17A) and Interleukin 17F(IL-17F). This unique mechanism of action may offer better therapeutic effects compared with other IL-17A class drugs. Bimekizumab was approved in August 2021 in the European Union and the United Kingdom for adult patients with moderate to severe plaque psoriasis who are candidates for systemic therapy, and in January 2022 in Japan for the treatment of adult patients with plaque psoriasis, generalized pustular psoriasis, and erythrodermic psoriasis who are inadequately responsive to existing therapies. Subsequently, in February and March 2022, it received approval in Canada and Australia. This recent approval of bimekizumab in the United States is expected to provide a new treatment option for even more patients.
The efficacy and safety of bimekizumab were evaluated in three phase 3 trials compared to the placebo and the IL-12/IL-23 inhibitor ustekinumab (BE VIVID trial), the placebo alone (BE READY trial), and the TNFα antibody adalimumab (BE SURE trial). All studies met their primary and secondary endpoints. Compared to patients treated with the active comparator (secondary endpoint, BE VIVID; p<0.0001), placebo (primary endpoint, BE READY and BE VIVID; p<0.0001) and adalimumab (primary endpoint, BE SURE; p<0.001), in the 16th week, patients treated with bimekizumab showed significantly improved skin clearance rates, expressed as PASI 90 (Psoriasis Area and Severity Index improvement of at least 90%) and investigator's global assessment (IGA) scores of 0 (clear of psoriasis symptoms) or 1 (almost clear).
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
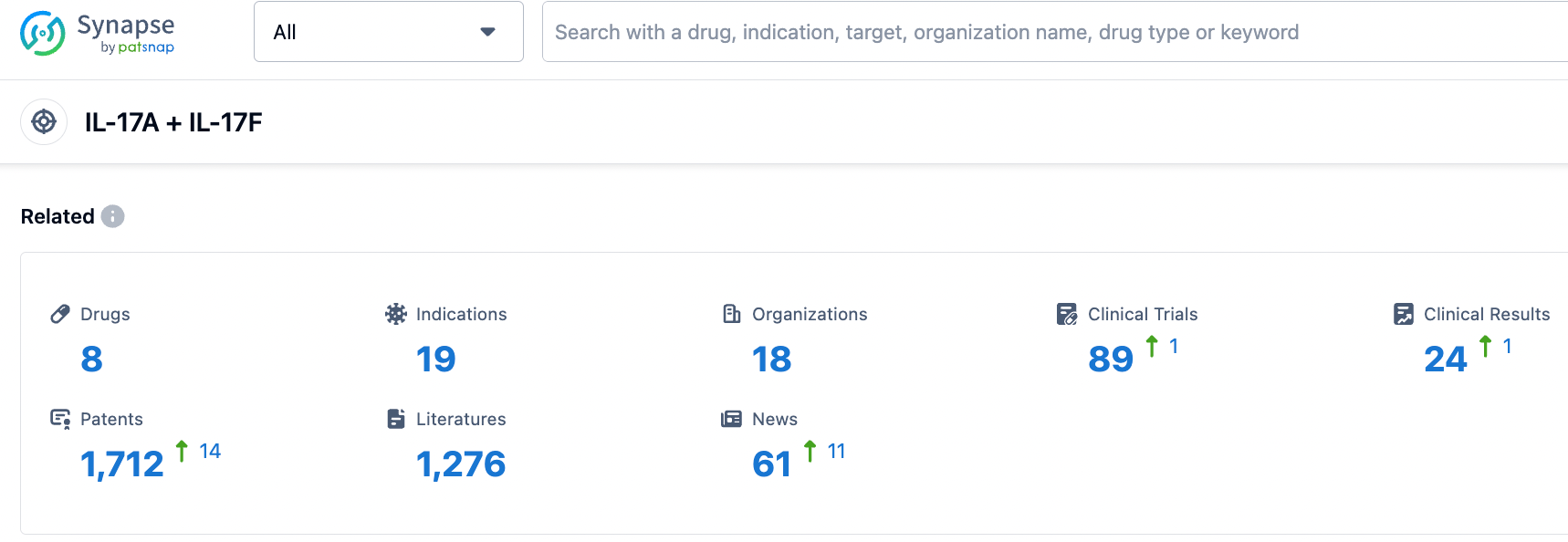
According to the information disclosed by the synapse database, as of October 20, 2023, there are eight drugs in development targeting IL-17A/F, with 19 indications covered. Eighteen institutions are involved in the research, with 89 related clinical trials and as many as 1711 patents. We look forward to more drugs being approved, bringing new treatment options to patients worldwide.