Analysis on the Clinical Research Progress of β-lactamase inhibitor
β-lactamase is an enzyme found in the human body that plays a crucial role in the defense against bacterial infections. It is responsible for breaking down β-lactam antibiotics, a class of drugs commonly used to treat bacterial infections. By targeting and degrading these antibiotics, β-lactamase helps bacteria develop resistance mechanisms, making them less susceptible to treatment. This enzyme is produced by certain bacteria as a defense mechanism, allowing them to survive and multiply in the presence of β-lactam antibiotics. Understanding the role of β-lactamase is essential in developing strategies to combat antibiotic resistance and improve the effectiveness of antibiotic therapies.
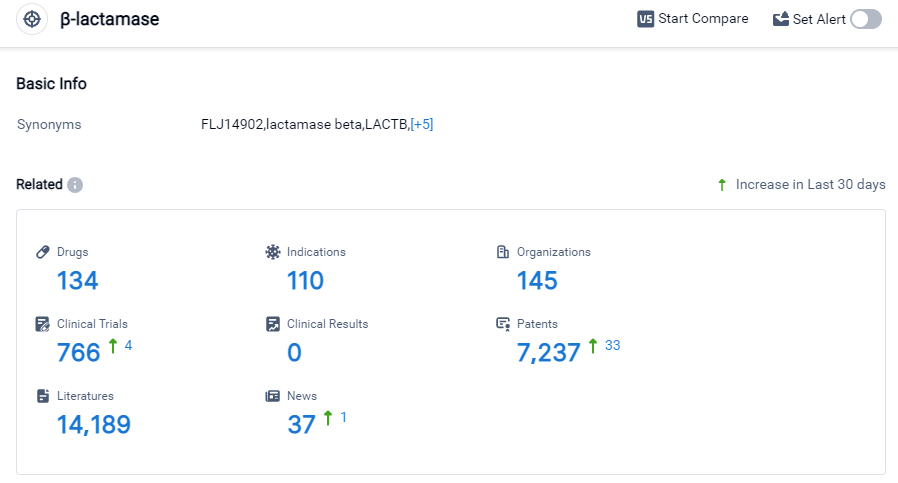
β-lactamase Competitive Landscape
According to Patsnap Synapse, as of 27 Sep 2023, there are a total of 134 β-lactamase drugs worldwide, from 145 organizations, covering 110 indications, and conducting 766 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
The analysis of the target β-lactamase in the pharmaceutical industry reveals a competitive landscape with multiple companies actively developing drugs. Pfizer Inc. and Merck & Co., Inc. are leading in terms of the number of drugs in various development phases. Bacterial infections are the most common approved indication for drugs targeting β-lactamase. Small molecule drugs dominate the drug type analysis, indicating intense competition and potential for biosimilars. China is the country with the highest number of approved drugs, indicating significant progress in the field. Overall, the target β-lactamase presents a promising area for future development in the pharmaceutical industry.
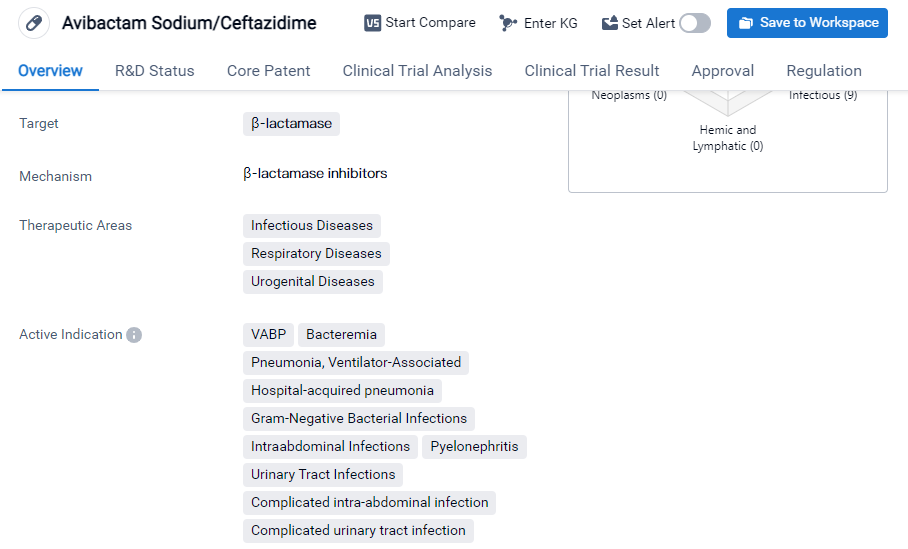
Key drug: Avibactam Sodium/Ceftazidime
Avibactam Sodium/Ceftazidime is a small molecule drug that targets β-lactamase, an enzyme responsible for antibiotic resistance in bacteria. It is primarily used in the treatment of infectious diseases, respiratory diseases, and urogenital diseases. The drug has been approved for various indications, including ventilator-associated pneumonia, bacteremia, hospital-acquired pneumonia, gram-negative bacterial infections, intraabdominal infections, pyelonephritis, etc.
The originator organization of Avibactam Sodium/Ceftazidime is Allergan Ltd., based in Ireland. The drug has achieved the highest phase of approval both globally and in China. It received its first approval in the United States in February 2015. The regulatory status of Avibactam Sodium/Ceftazidime includes priority review and fast track, indicating that it has undergone expedited evaluation processes due to its potential to address unmet medical needs.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Avibactam Sodium/Ceftazidime is a significant development in the field of biomedicine, particularly in the fight against antibiotic resistance. By targeting β-lactamase, it helps to overcome one of the major mechanisms through which bacteria develop resistance to antibiotics. This drug offers a promising solution for the treatment of various infectious diseases caused by gram-negative bacteria.
The approval of Avibactam Sodium/Ceftazidime in multiple indications highlights its broad therapeutic potential. It can be used to treat serious infections such as pneumonia, bacteremia, and urinary tract infections, including complicated cases. The drug's approval in the United States and China, two major pharmaceutical markets, further emphasizes its global significance.
The regulatory designations of priority review and fast track indicate that Avibactam Sodium/Ceftazidime has undergone an accelerated evaluation process. This suggests that regulatory authorities recognize the urgent need for effective treatments in the indicated therapeutic areas. The fast track designation also implies that the drug has demonstrated promising results in early clinical trials, warranting expedited development and review.
In summary, Avibactam Sodium/Ceftazidime is a small molecule drug developed by Allergan Ltd. It targets β-lactamase and has been approved for various indications in the treatment of infectious diseases, respiratory diseases, and urogenital diseases. Its approval in the United States and China, along with priority review and fast track designations, highlights its potential to address unmet medical needs and combat antibiotic resistance.