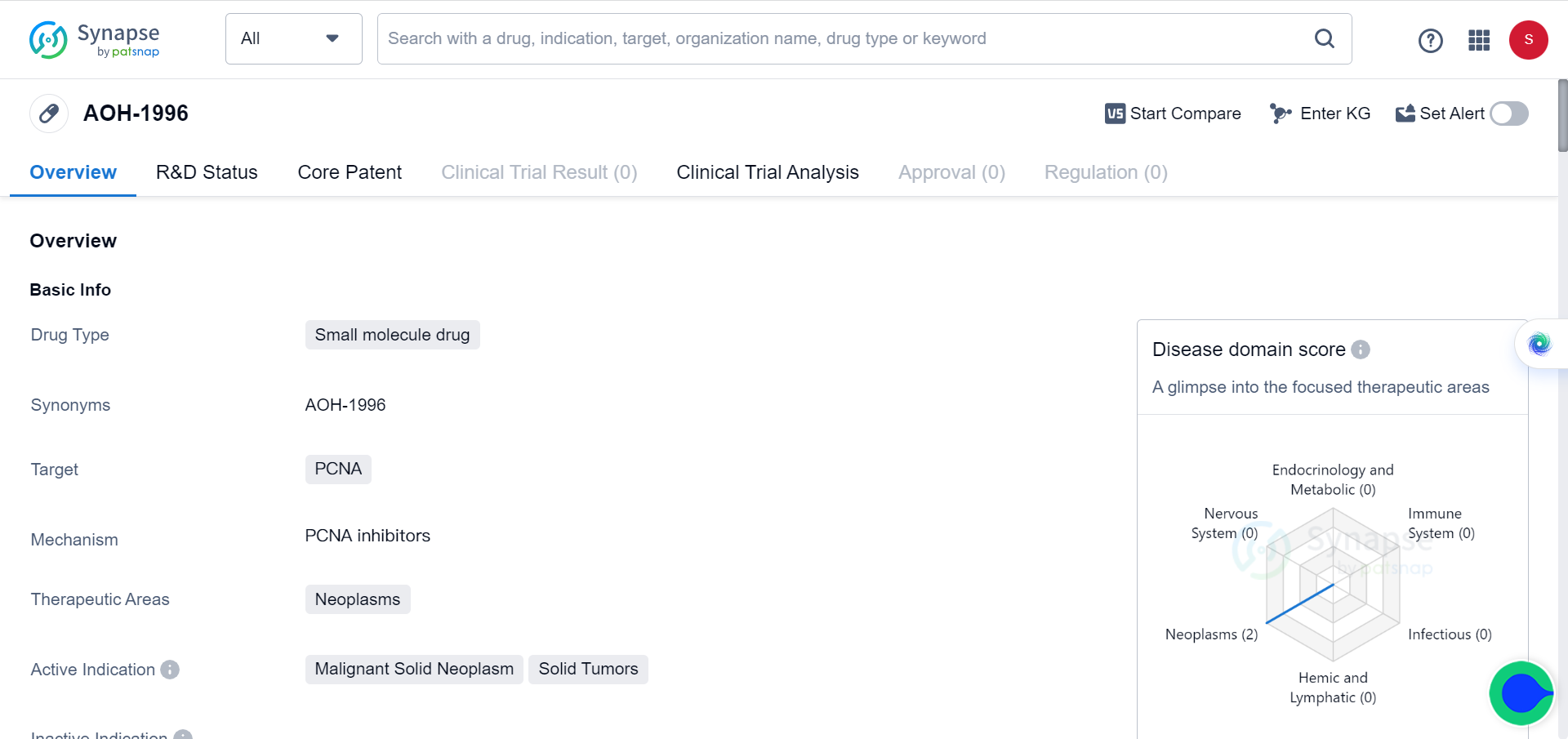
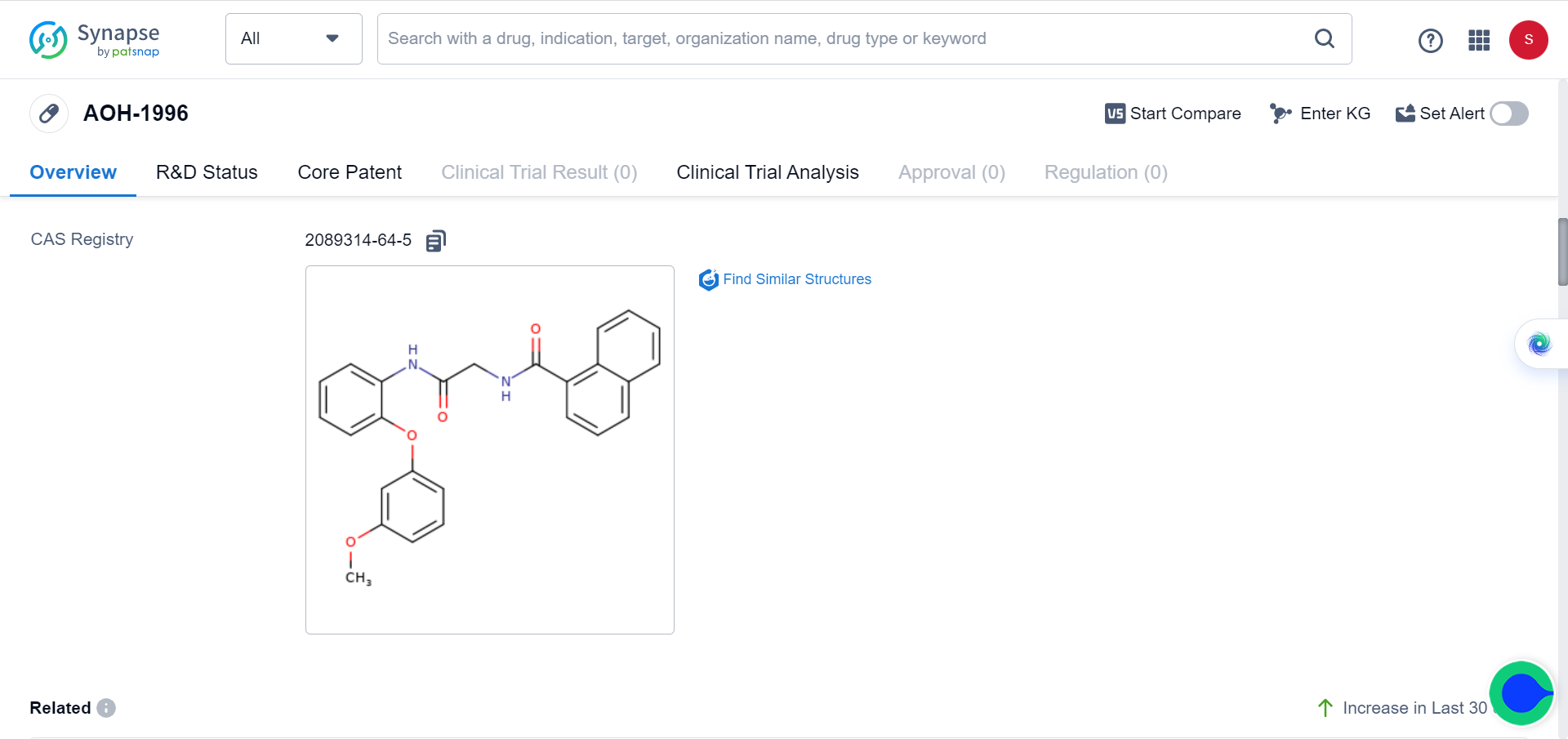
AOH1996: The Ultimate Weapon to Annihilate All Solid Tumours
Proliferating Cell Nuclear Antigen (PCNA) is a protein present in all eukaryotic cells that plays a crucial role in DNA synthesis and repair. It is widely used as a biomarker for tumor progression and is also considered a potential target for cancer therapy.
"Holy grail" molecule
Recently, a team from City of Hope in California, United States, published a study showing that a new type of anticancer drug targeting PCNA can eliminate all solid tumors. This drug, called AOH1996, hailed as ‘holy grail’ molecule by many, has been shown to be effective in preclinical studies of breast cancer, prostate cancer, brain cancer, ovarian cancer, cervical cancer, skin cancer, and lung cancer cells.
The study, titled "Small molecule targeting of transcription-replication conflict for selective chemotherapy," was published in the Cell Chemical Biology journal. The new potential anti-cancer drug that targets genomic instability in cancer cells shows promise as an oral treatment option with few side effects.
Cancer cells are known to have elevated levels of DNA damage and suffer from defects in DNA repair mechanisms, resulting in genomic instability. This instability creates an opportunity for drugs to selectively target cancer cells. In their study, the researchers honed in on transcription-replication conflicts, a significant source of genomic instability in cancer, as the key focus of their investigation.
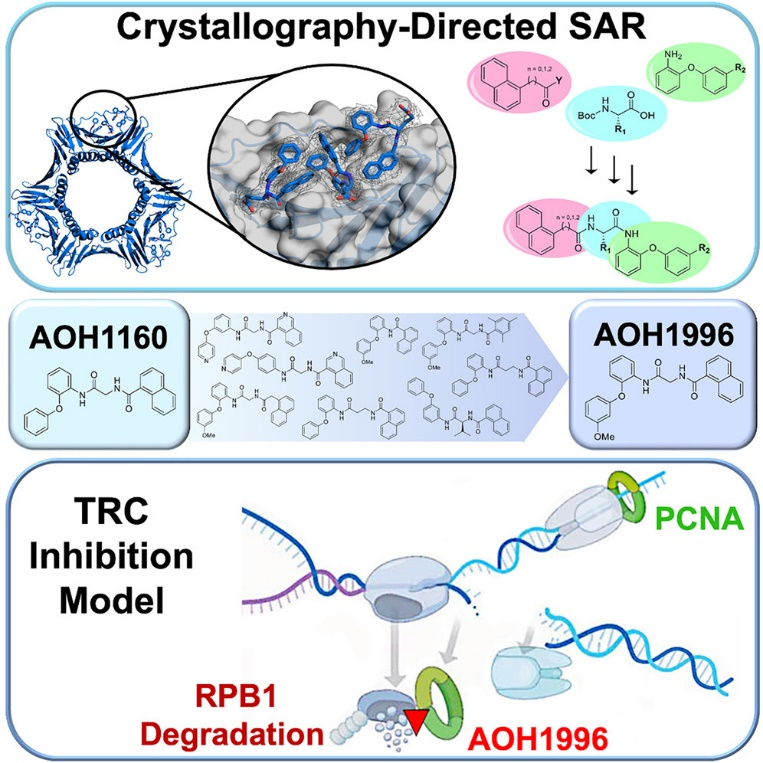
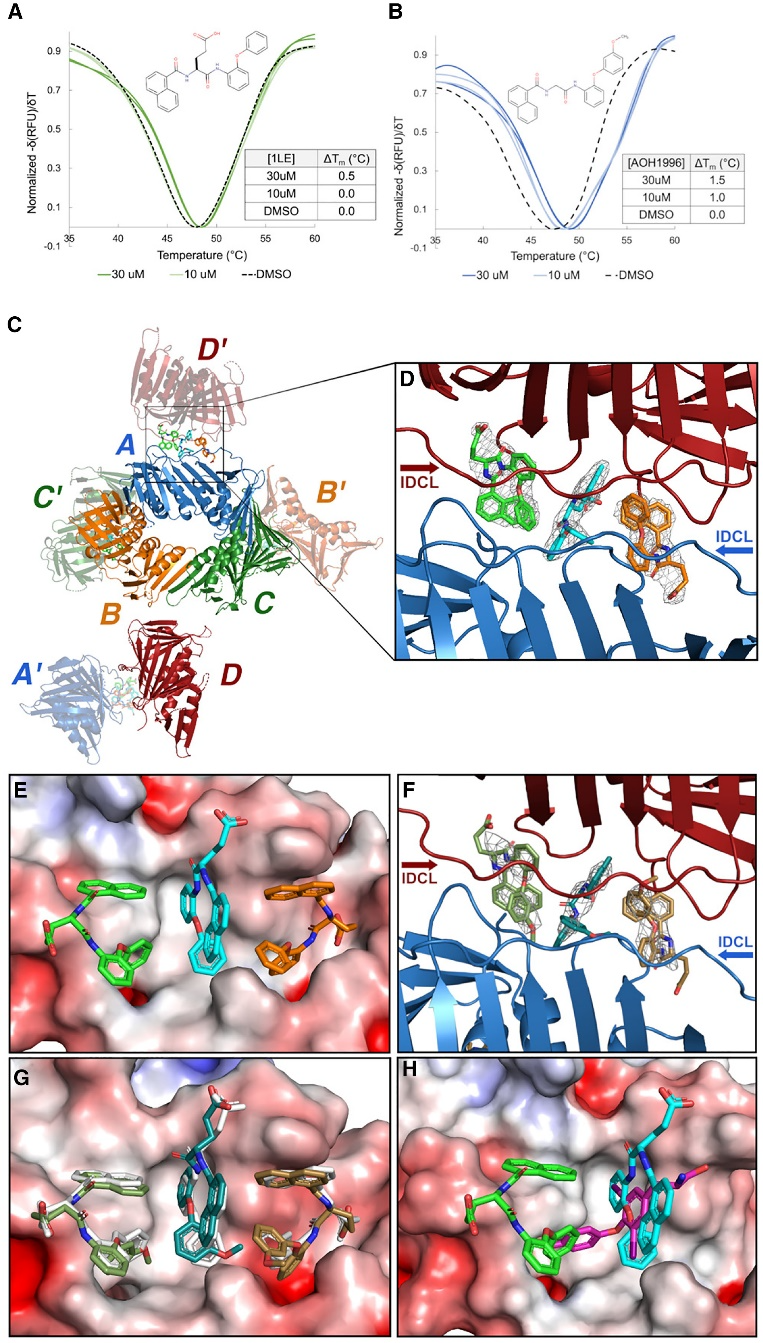
They targeted PCNA through medicinal chemistry approaches and showed that AOH1996 binds to PCNA and inhibits its interaction with RNA polymerase II, a key protein involved in transcription.
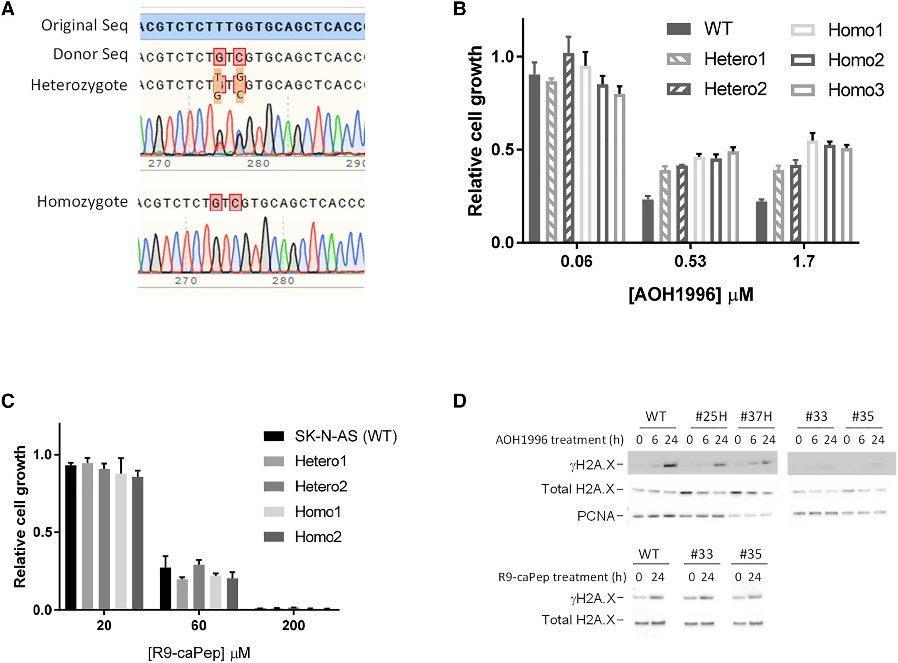
When tested in cancer cell lines, AOH1996 selectively killed cancer cells while having little effect on normal cells. It induced DNA double-strand breaks, cell cycle arrest and apoptosis in cancer cells. It also sensitized cancer cells to other anti-cancer drugs like cisplatin and topotecan.
In mouse models of human cancer, daily treatment with the drug AOH1996 considerably slowed tumor growth with no noticeable side effects. The highest dose that did not cause adverse effects was more than 6 times the effective dose in mice and dogs. Researchers intend to further examine the drug's distribution within the body, activity, and safety profile to ascertain its suitability for human testing.
If clinical trials are positive, AOH1996 may present a novel option for some cancer patients by acting on a molecular defect while possibly resulting in fewer side effects compared to current treatments. The drug selectively attacked the cancer cells, leaving normal cells unharmed.
Clinical trials underway
Lead author Linda Malkas, a professor in the Molecular Diagnostic and Experimental Therapeutics Department at City of Hope, said the results of the cell and animal models were encouraging, and Phase I clinical trials are currently underway. "AOH1996 can inhibit tumor growth as a monotherapy or combination therapy in cell and animal models without causing toxicity," she said.
Long Gu, the study's main author, said that no one had previously considered PCNA as a therapeutic target because it was considered "undruggable."
According to a statement from City of Hope, the first patient treated with AOH1996 is in good condition. Phase I clinical trials are being conducted at City of Hope in Los Angeles to determine the maximum tolerated dose of the drug and evaluate its preliminary efficacy.
During DNA replication, replication stress caused by extracellular and intracellular factors can lead to replication fork stalling or termination. If cells cannot respond appropriately to this risk, chromosomal breaks and rearrangements can occur, leading to genomic instability.
AOH1996 was named after Anna Olivia Healy, who was born in 1996 and was diagnosed with a rare childhood cancer, neuroblastoma, at the age of nine. She passed away later on.
Linda Malkas, the head of the team, has been working on the development and research of anticancer drugs for the past 20 years. The team used computer modeling to simulate molecular interactions with the L126-Y133 region of PCNA. The model simulated molecular interactions of key proteins involved in DNA replication. After multiple simulations and analysis, the team discovered AOH1996, which showed potential to inhibit PCNA activity, thereby disrupting DNA replication in cancer cells and inducing cell death.
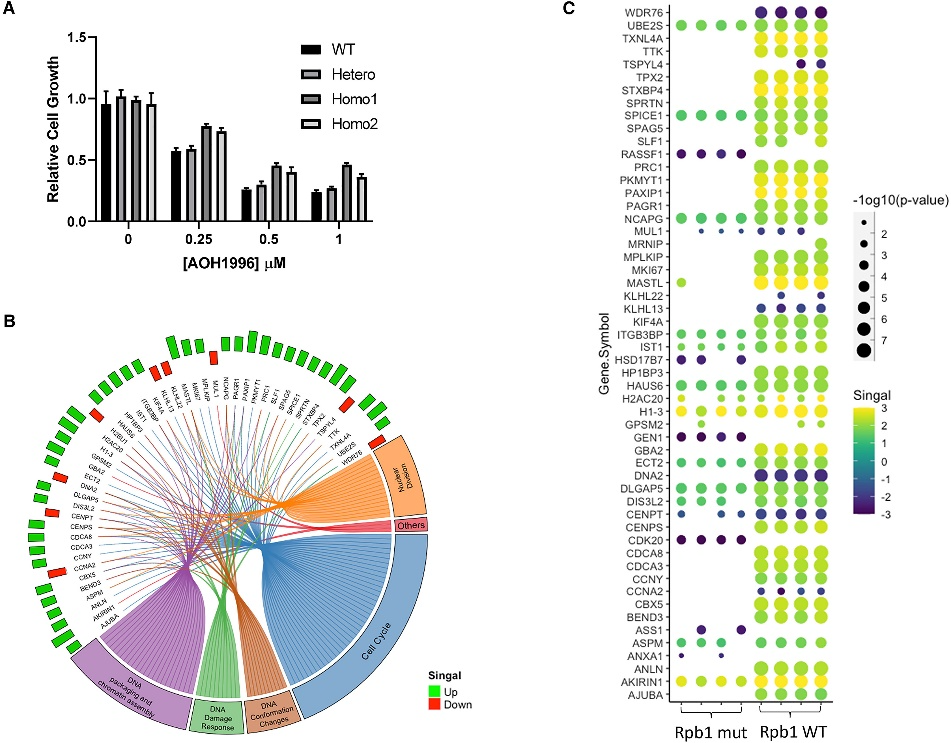
In their experiments, the researchers tested AOH1996 on more than 70 cancer cell lines and several normal control cells. They discovered that AOH1996 specifically targets cancer cells by disrupting the normal cell cycle.
What's even more intriguing is that the study found that AOH1996 increases the vulnerability of cancer cells to chemical agents that cause DNA or chromosome damage, such as the chemotherapy drug cisplatin. This implies that AOH1996 could be an effective tool for combination therapy and the development of new chemotherapy drugs.
As of now, the exclusive license for AOH1996 has been granted to RLL, LLC by City of Hope, a biotechnology company co-founded by Malkas.
Reference
Gu et al., Small molecule targeting of transcription-replication conflict for selective chemotherapy, Cell Chemical Biology (2023).