Moderna Pipeline Review — Progress of 83 drug developments by the pioneer of mRNA vaccines
Moderna, a developer of mRNA drugs and vaccines, was established in 2010 and is headquartered in Cambridge, Massachusetts, USA, being a rising company in the field of biotechnology.
Development History
In the early days of its establishment, Moderna carried out a series of research and development projects for conventional vaccines, trying to dominate this vast and profitable market with new vaccines.
However, most of these research and clinical trials ended in failure. Nevertheless, through this process of innovation, Moderna firmly established its leading position in the mRNA field, attracting the attention of many pharmaceutical companies.
In 2013, Moderna partnered with AstraZeneca to enter the field of therapeutic vaccines.
In 2014, Moderna joined hands with Alexion to enter the field of rare diseases.
The outbreak of the COVID-19 pandemic in 2020 gave Moderna an opportunity. In the financial year 2021, Moderna successfully reversed years of losses with just one commercial product, Spikevax, with a net profit of $12.2 billion, an increase of 1733.33% over the same period last year.
However, as the curtain gradually closes on the COVID-19 pandemic, how to produce the next blockbuster has become a problem that Moderna inevitably has to consider.
Pipeline Layout
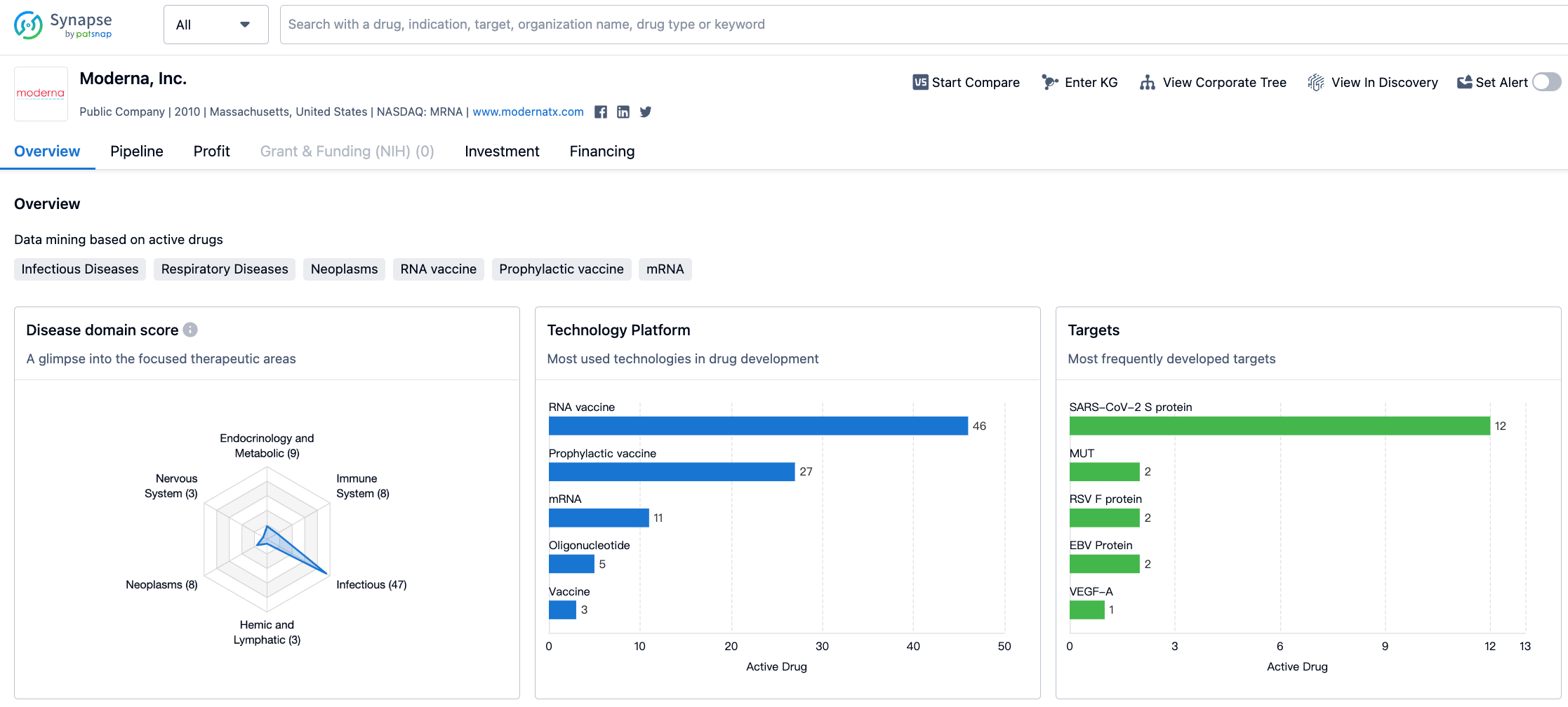
Moderna's R&D pipeline covers multiple areas, with major research areas including COVID-19 vaccines, influenza vaccines, preventive vaccines, systemic secretion and cell surface treatments; exploratory areas include tumor vaccines, local regeneration therapies, cell therapies and more.
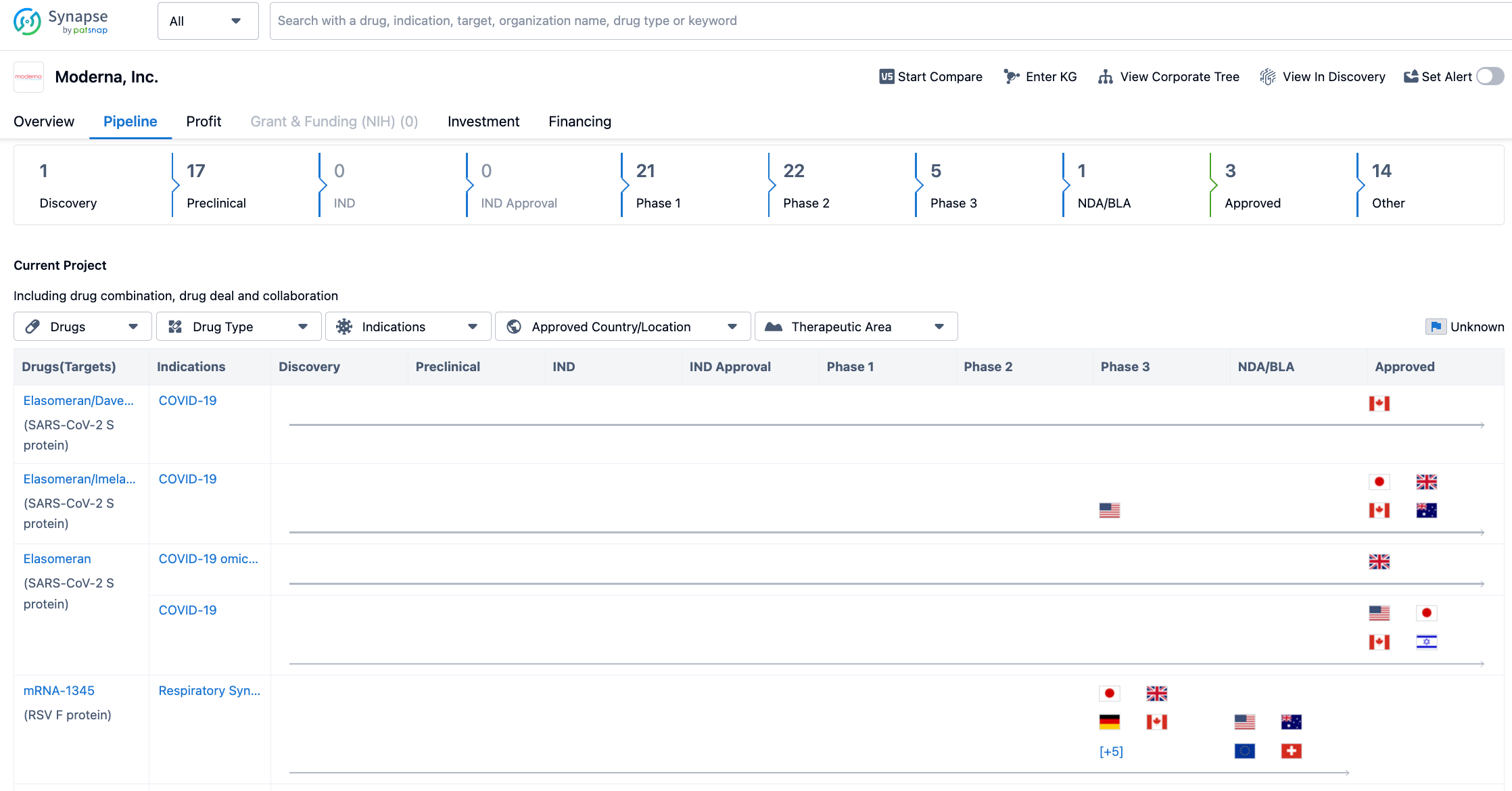
As of August 2, 2023, Moderna's drug pipeline status is as follows: 1 drug at the drug discovery stage, 17 drugs at the preclinical stage, 21 drugs at phase 1 clinical trials, 22 drugs at phase 2 clinical trials, 5 drugs at phase 3 clinical trials, 1 drug at the application for marketing stage, and 3 drugs at the approved for marketing stage.
Most of the drugs are focused on the infectious diseases area, with the COVID-19 vaccine being the most prominent.
Among them, so far, Moderna has been approved to sell three COVID-19 vaccines under the "Spikevax" brand, including:
1. A vaccine for the early original strain of SARS-CoV-2 (mRNA-1273);
2. A bivalent vaccine for the original strain and Omicron BA.1 (mRNA-1273.214);
3. A bivalent vaccine for Omicron BA.4/BA.5 (mRNA-1273.222).
The RSV vaccine mRNA-1345 is at the stage of application for marketing. In January 2023, the FDA awarded mRNA-1345 the title of "Breakthrough Therapy" for the prevention of lower respiratory tract diseases caused by RSV in adults aged 60 or over; it had previously been awarded the "Fast Track" title by the FDA in August 2021.
Among the projects in phase three clinical trials, there is a new COVID-19 vaccine mRNA-1283 developed for the variant virus, the influenza vaccine mRNA-1010, and the cytomegalovirus (CMV) vaccine mRNA-1647. The projects in phase 2 clinical trials include the public health vaccine Zika (Zika) vaccine mRNA-1893 and the individualized new antigen therapy (INT) vaccine mRNA-4157, etc.
As Moderna's mRNA delivery technology becomes more mature, several vaccines use the same delivery method as the COVID-19 vaccine. As the interest in the new coronavirus decreases, perhaps Moderna will gradually shift its focus towards other diseases while continuing to promote research and development of vaccines for new coronavirus variant strains, bringing hope of recovery to more patients with autoimmune diseases, rare diseases, and tumors.