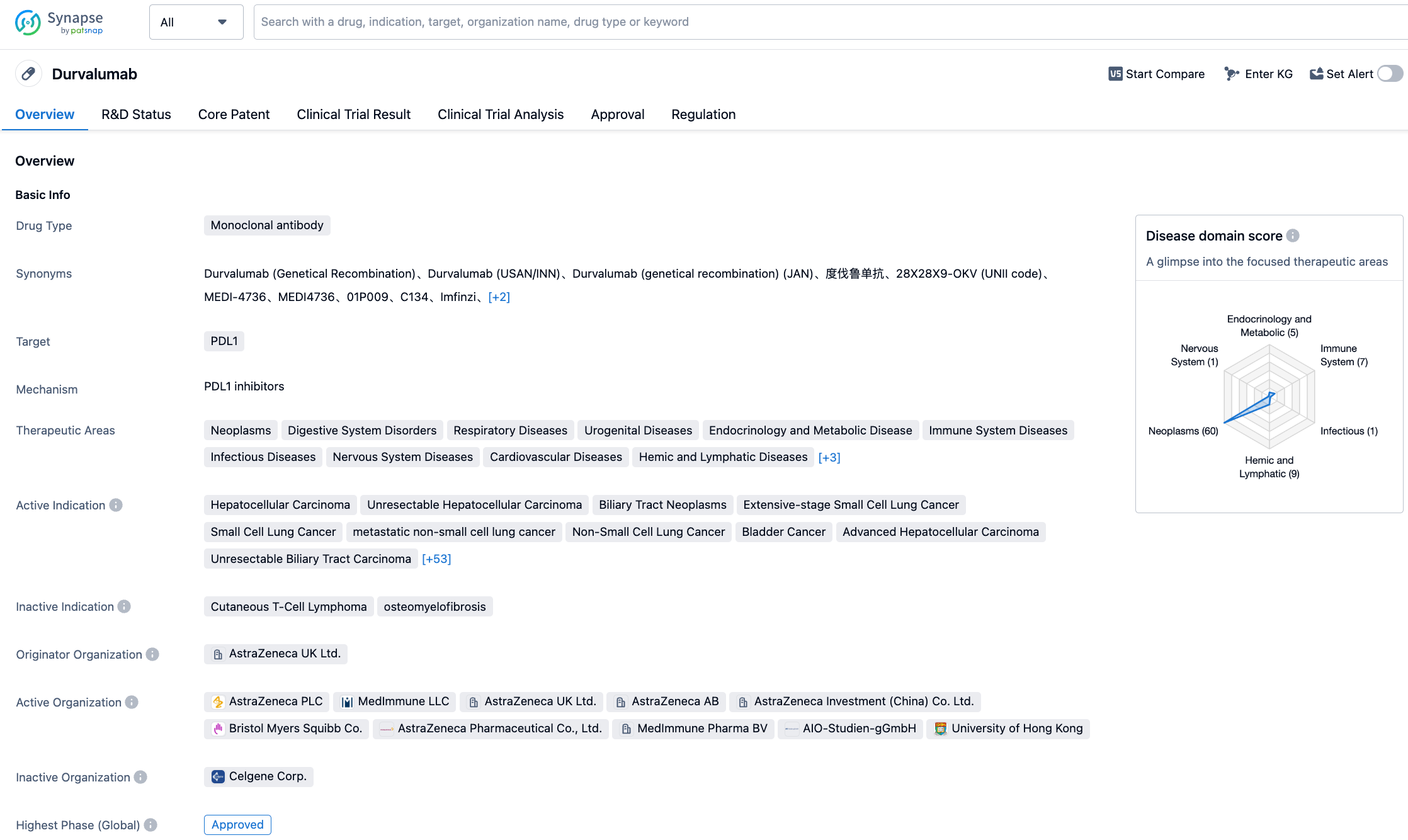
AstraZeneca has agreed to pay Bristol-Myers Squibb $510 million to resolve patent disputes
On August 1, 2023, AstraZeneca has reached an agreement to pay $510 million to BMS to resolve patent lawsuit over its PD-L1 mAb Imfinzi (Durvalumab) and CTLA-4 mAb Imjudo (Tremelimumab).
In March 2022, BMS first filed lawsuits claiming that Imfinzi (Durvalumab) infringed at least 8 patents related to Opdivo (Nivolumab), and added claims of PD-1 patent infringement in another lawsuit in April. In January, just three months after Imjudo (Tremelimumab) went on the market, BMS filed another lawsuit against AstraZeneca for infringing on patents related to Yervoy (Ipilimumab). AstraZeneca spokesperson said the company chose to spend $510 million to resolve all patent disputes with BMS and its partner Ono Pharmaceutical over the two drugs. According to AstraZeneca’s 2023 H1 financial report, Imfinzi had sales of $1.976 billion in the first half of the year, up 57% YoY; Imjudo also has good market potential. It is a wise decision to pay patent fees in order to facilitate subsequent commercialization of the two drugs.
For pharmaceutical companies, antibodies targeting PD-1/PD-L1 would likely struggle to avoid patents held by BMS and Ono Pharmaceutical if they seek to sell in markets such as the USA and Europe. Almost any use of antibody drugs related to PD-1/PD-L1 immune suppressant signals will infringe on patents held by BMS and Ono. Prior to AstraZeneca, pharmaceutical companies including Merck, Roche, and others chose to settle with BMS. Merck’s settlement agreement with Ono stipulates that from 2017 to 2023, Merck must pay a 6.5% sales split on Keytruda. From 2024 to 2026, the payment percentage drops to 2.5%. BMS and Ono split the additional revenue equally in a 3:1 ratio. Roche also spent a large sum of money for settlement. In 2020, BMS, Ono and Roche agreed on a global patent license agreement for Roche’s anti-PD-L1 antibody Tecentriq. According to the agreement, Roche paid $324 million, with additional annual single-digit royalties on worldwide net sales until 2026.
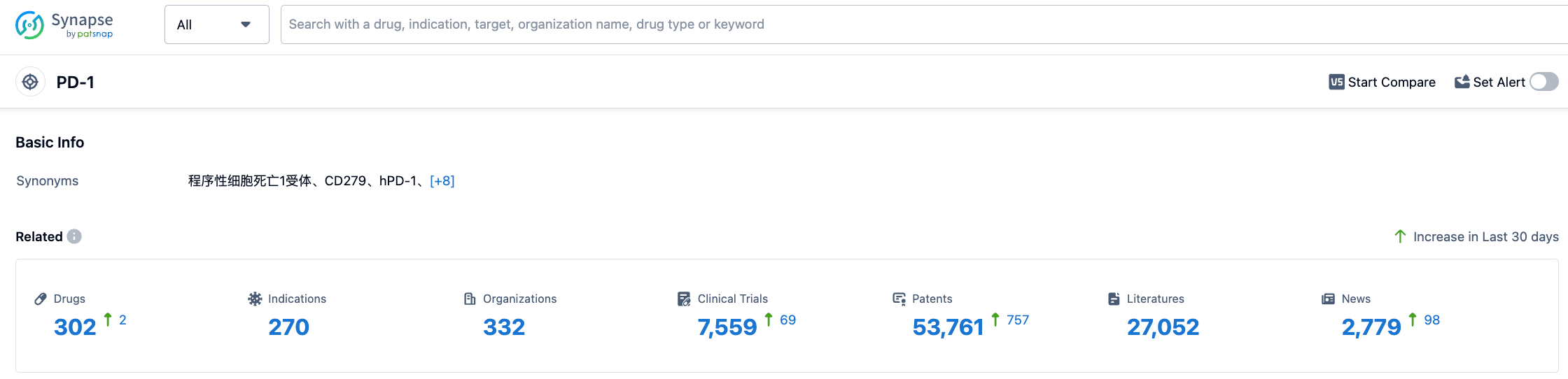
According to information disclosed by Synapse (click the card below to access the PD-1 target login after registration to get free detailed information on the drug under this target, indication, R&D agency, clinical trials, etc.), as of August 3, 2023, there are 302 drugs under development targeting PD-1, including 270 indications, 332 R&D agencies, with 7556 related clinical trials, and up to 53789 patents…PD-1/PD-L1 is one of the hottest areas of R&D, and has successively produced numerous blockbuster drugs, including the $20 billion sales of Keytruda. With their related patents, BMS and Ono can collect substantial fees, demonstrating the importance of proper patent layout for new drug development.