April 2023 Global Innovative Drug Report
This April 2023 Global Innovative Drug Report, based on data from the PatSnap Synapse database, offers a comprehensive overview of the latest advancements in the pharmaceutical industry. Divided into four parts, it examines the R&D status, active patents, mechanisms of action, clinical trials, competitive landscape, and other pertinent factors of each selected drug.
Download the report to gain valuable insights into:
1.The first approved drugs in April
2.Comprehensive analysis of newly introduced medications
3.Global drugs under Expedited Review Pathway in April
4.In-depth analysis of selected ERP drugs
Global Innovative Drug Report Overview
1.) First Approved Drugs in April
In April 2023, a total of 8 drugs received global approval, encompassing a diverse range of therapeutic categories. These notable approvals consisted of 2 Prophylactic vaccines, 2 Small molecule drugs (SMDs), 1 Monoclonal antibody (mAb), 1 Hematopoietic stem cell treatment, 1 Oligonucleotide antisense therapy, and 1 Live biotherapeutic product.

2.) Comprehensive analysis of newly introduced medications
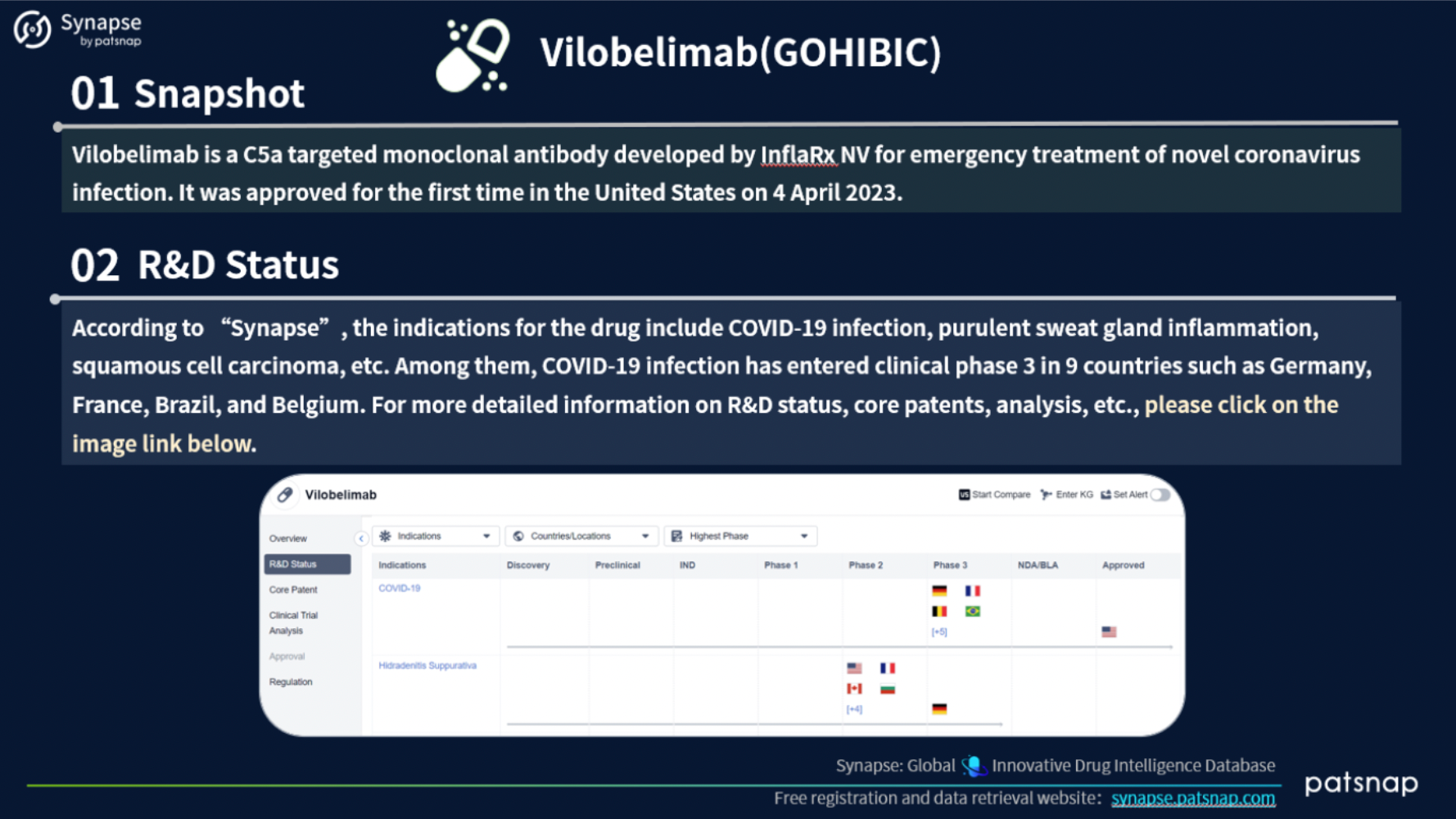
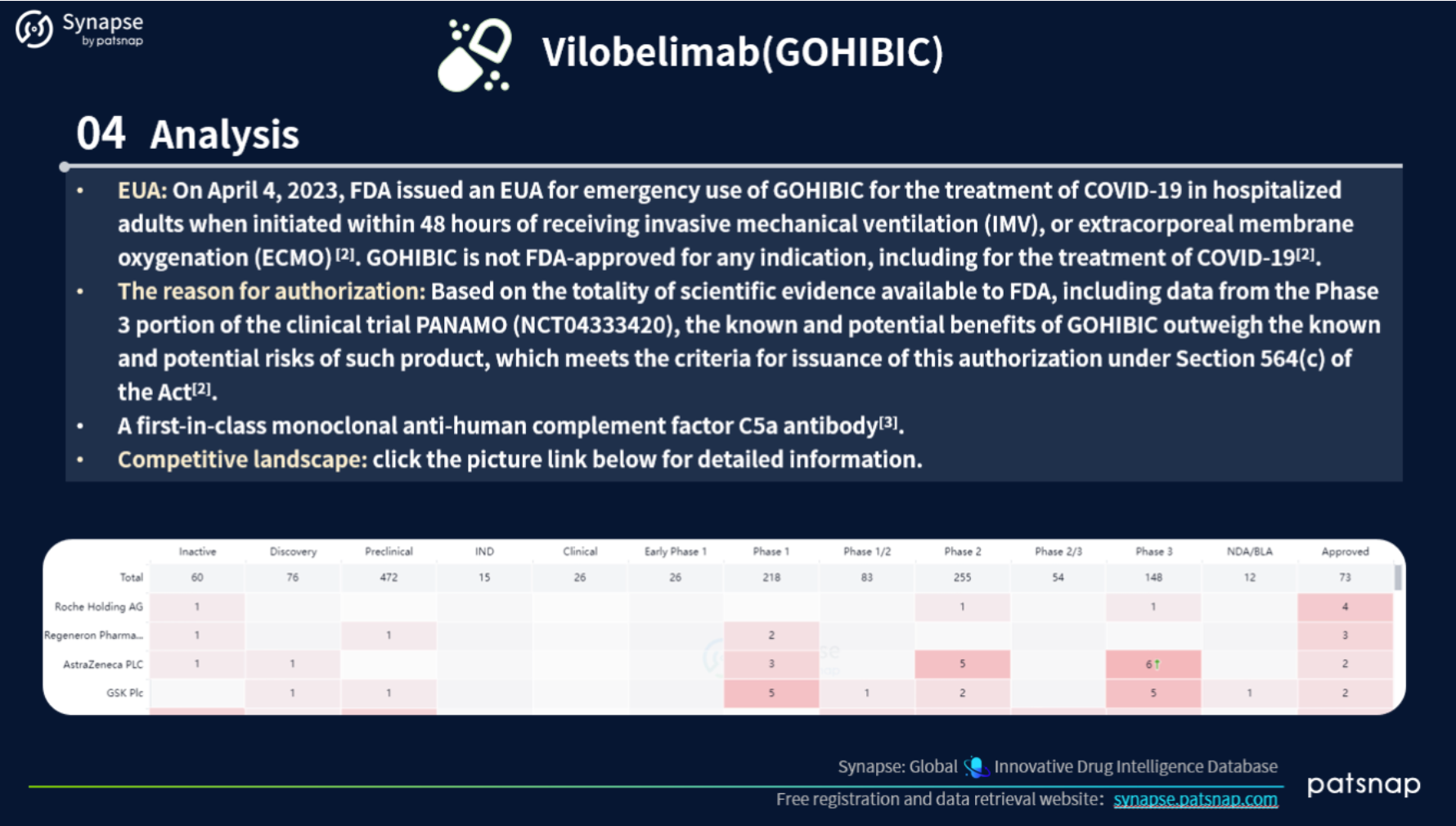
This report highlights 5 recently approved drugs for an in-depth analysis. Let’s take a closer look at the report content for Vilobelimab, which includes the following comprehensive details:

3.) Global drugs under Expedited Review Pathway in April
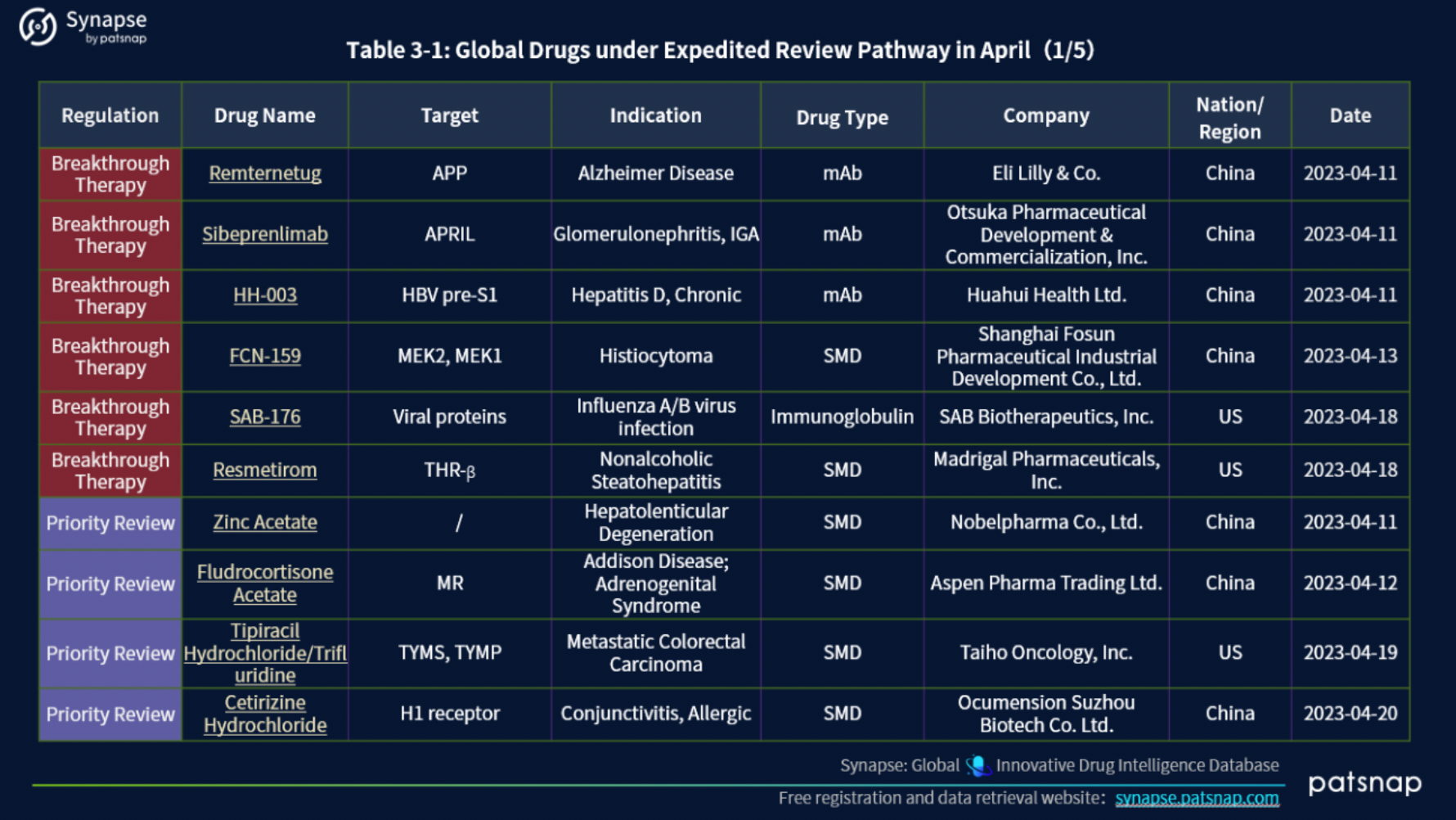
In April 2023, 45 drugs underwent Expedited Review pathways (ERP). This cohort includeds a diverse range of designations, with 16 drugs holding the esteemed Orphan Drug designation, 12 designated as Fast Track, 6 deemed Breakthrough Therapy, 4 granted Priority Review, 2 bestowed with the Rare Pediatric Disease designation, 2 under the Innovative Licensing and Access Pathway (ILAP), 1 marked for Accelerated Approval, 1 authorized for Emergency Use, and 1 falling under the PRIME (EU) program.
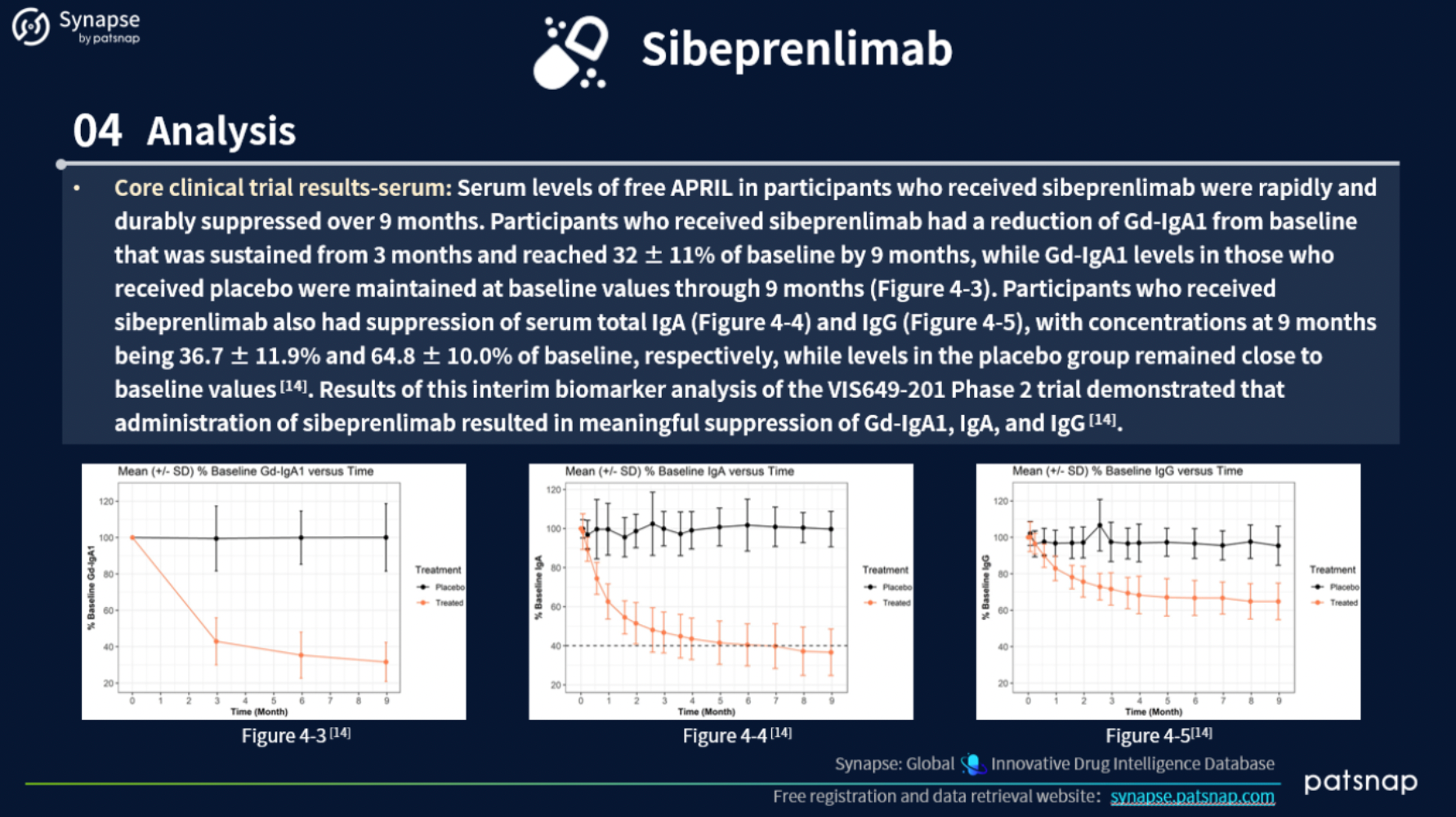
4.) In-depth analysis of selected ERP drugs
For this report, we selected 6 ERP drugs to analyze. Let’s delve into the report content for Sibeprenlimab, which presents the following in-depth details:


Gain Access to the Comprehensive Report for FREE – Download Now! If you aren’t registered for Synapse (registration is required to download the report), click here to register for free.

Copyright Statement: This report is the sole property of PatSnap and is protected under copyright laws. Any reproduction, excerpting, or other use of this report without explicit authorization from PatSnap is strictly prohibited. Authorized products must be used within the scope of authorization and must include a clear indication of the source. PatSnap reserves the right to investigate any violations of this statement and pursue legal action as necessary. For inquiries regarding authorization, please contact phs@patsnap.com.