AriBio Granted EMA Approval for Phase 3 Trials of AR1001 in Alzheimer’s Treatment (POLARIS-AD)
AriBio Co., Ltd. has received approval from the European Medicines Agency (EMA) for POLARIS-AD (AR10011-ADP3-US01), a phase 3 global clinical trial targeting early Alzheimer's disease. This approval brings EMA into agreement with its counterparts, the U.S. Food and Drug Administration (FDA) and the UK's Medicines and Healthcare Products Regulatory Agency (MHRA), regarding the Clinical Trial Authorisation for POLARIS-AD.
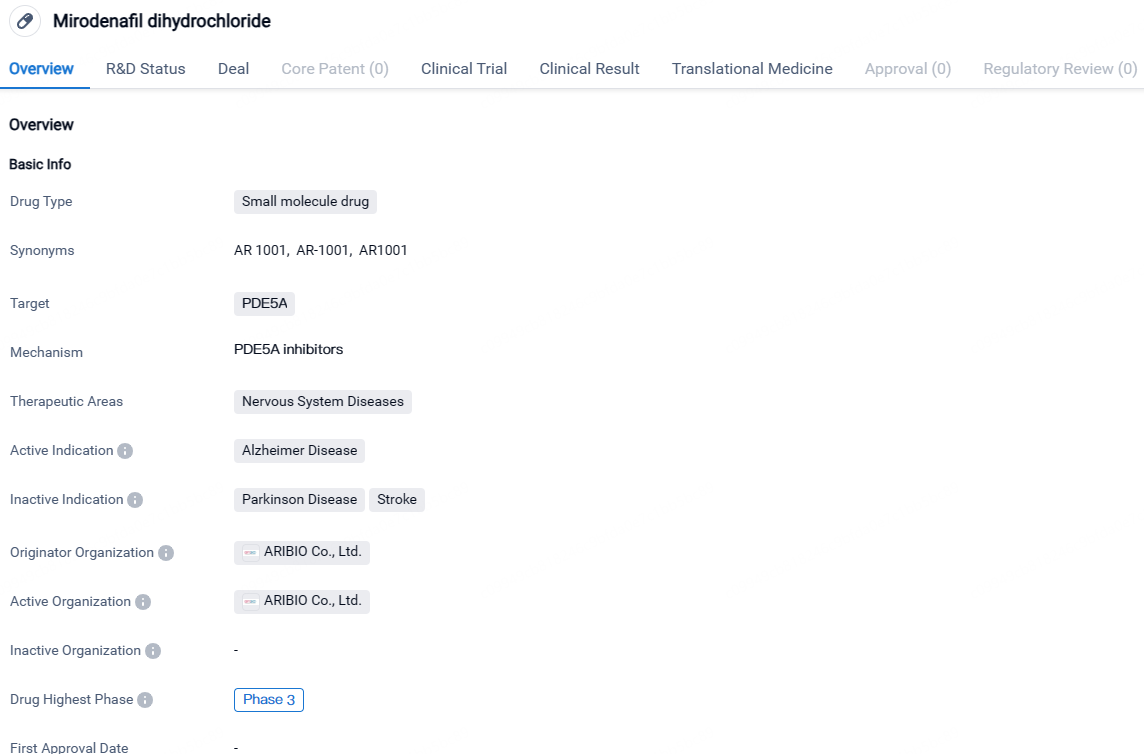
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
On April 24th, the EMA finalized its decision, granting clinical trial authorizations for the following member countries: Czech Republic, Denmark, France, Germany, Italy, Spain, and The Netherlands. At present, it is anticipated that 1,150 subjects will be enrolled in a Phase 3 clinical trial at 200 locations worldwide.
POLARIS-AD is a multi-center, double-blind, placebo-controlled, randomized registration trial, which is assessing the effectiveness and safety of AR1001 (Mirodenafil) in treating patients with early-stage Alzheimer’s disease who have confirmed amyloid pathology.
This study adopts the primary endpoint recognized by both the U.S. Food and Drug Administration and EMA, which is the Clinical Dementia Rating Scale–Sum of Boxes. It also includes several secondary endpoints: the Alzheimer’s Disease Assessment Scale-Cognitive Subscale 13, Amsterdam-Instrumental Activities of Daily Living Questionnaire, Geriatric Depression Scale, Mini-Mental Status Examination, and measurements of changes in cerebral spinal fluid and plasma biomarkers.
James Rock, Chief Clinical Officer at AriBio, commented, “With the endorsement of POLARIS-AD in the United Kingdom, extending our reach to additional European nations marks a significant progression in the licensing efforts for AR1001. This approval signifies the fourth territory initiating active screening and participant enrollment, set to commence from the second quarter of 2024.”
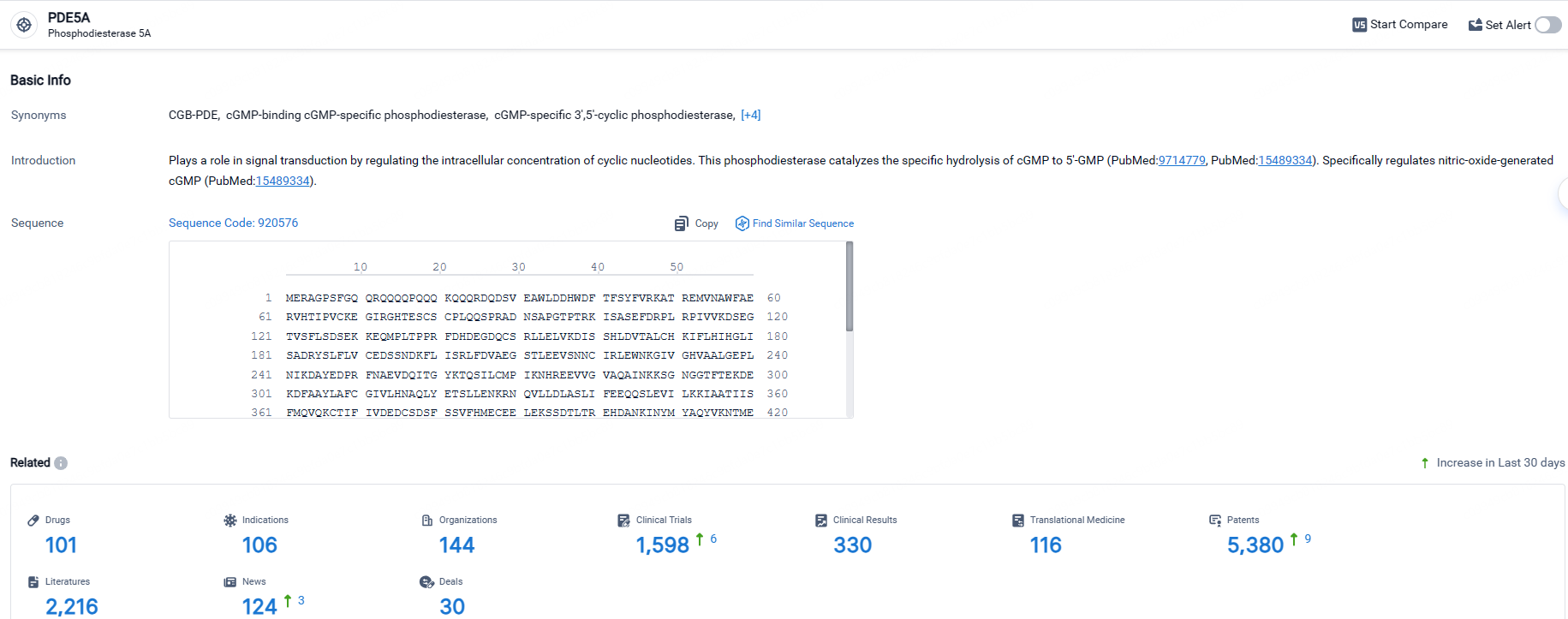
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of April 30, 2024, there are 101 investigational drugs for the PDE5 targets, including 106 indications, 144 R&D institutions involved, with related clinical trials reaching 1598, and as many as 5380 patents.
AR1001 is a phosphodiesterase-5 (PDE5) inhibitor being developed as an investigational oral agent for the treatment of Alzheimer’s disease. The drug has reached Phase 3 of clinical development globally and is currently at the IND application stage in China. The potential of Mirodenafil dihydrochloride to improve blood flow and neuronal function makes it a promising candidate for the treatment of Alzheimer's disease, a condition with limited therapeutic options.