Arrowhead Pharma Reveals Strong Gene Knockdown by ARO-RAGE in Asthma Patients
Arrowhead Pharmaceuticals, Inc. revealed fresh interim clinical findings regarding ARO-RAGE, an RNAi-based investigational drug targeting inflammatory lung conditions like asthma, at the American Thoracic Society 2024 International Conference. The preliminary outcomes from the ongoing Phase 1/2 trial indicate that administering ARO-RAGE resulted in a dose-dependent decrease in soluble RAGE levels in both bronchoalveolar lavage fluid and serum among healthy participants and individuals with mild to moderate asthma.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
“These findings highlight the potential of our exclusive TRiMTM platform in developing innovative treatments for various pulmonary disorders with significant unmet medical needs. Arrowhead was the pioneering company to demonstrate that RNA interference (RNAi) can be utilized to achieve substantial target gene reduction in the lungs of healthy human subjects, and the data shared at ATS 2024 build upon that earlier success,” stated James Hamilton, M.D., who is the chief of discovery and translational medicine at Arrowhead.
“The ARO-RAGE data are pioneering and exhibit profound and lasting gene knockdown in the lungs of patients with mild to moderate asthma. The duration of the effect supports a dosing regimen of once every two months, and the treatment has shown a favorable safety and tolerability profile. These results bolster our confidence as we prepare to launch a Phase 2 study in late 2024,” Hamilton added.
To date, ARO-RAGE has demonstrated a positive safety profile, with no observed patterns of systemic safety lab abnormalities or adverse impacts on lung function over time. There have been no serious adverse events linked to the study drug, nor have there been any treatment-related side effects leading to trial cessation.
Additionally, Arrowhead presented preclinical results for two other lung-targeted initiatives utilizing their proprietary Targeted RNAi Molecule platform. ARO-TSLP is designed to inhibit the cytokine thymic stromal lymphopoietin in the epithelium, a validated target that activates multiple immune cell lineages to drive asthmatic inflammation. ARO-IAV aims to silence the genetic expression of highly conserved influenza A viruses, including highly pathogenic strains such as avian influenza.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
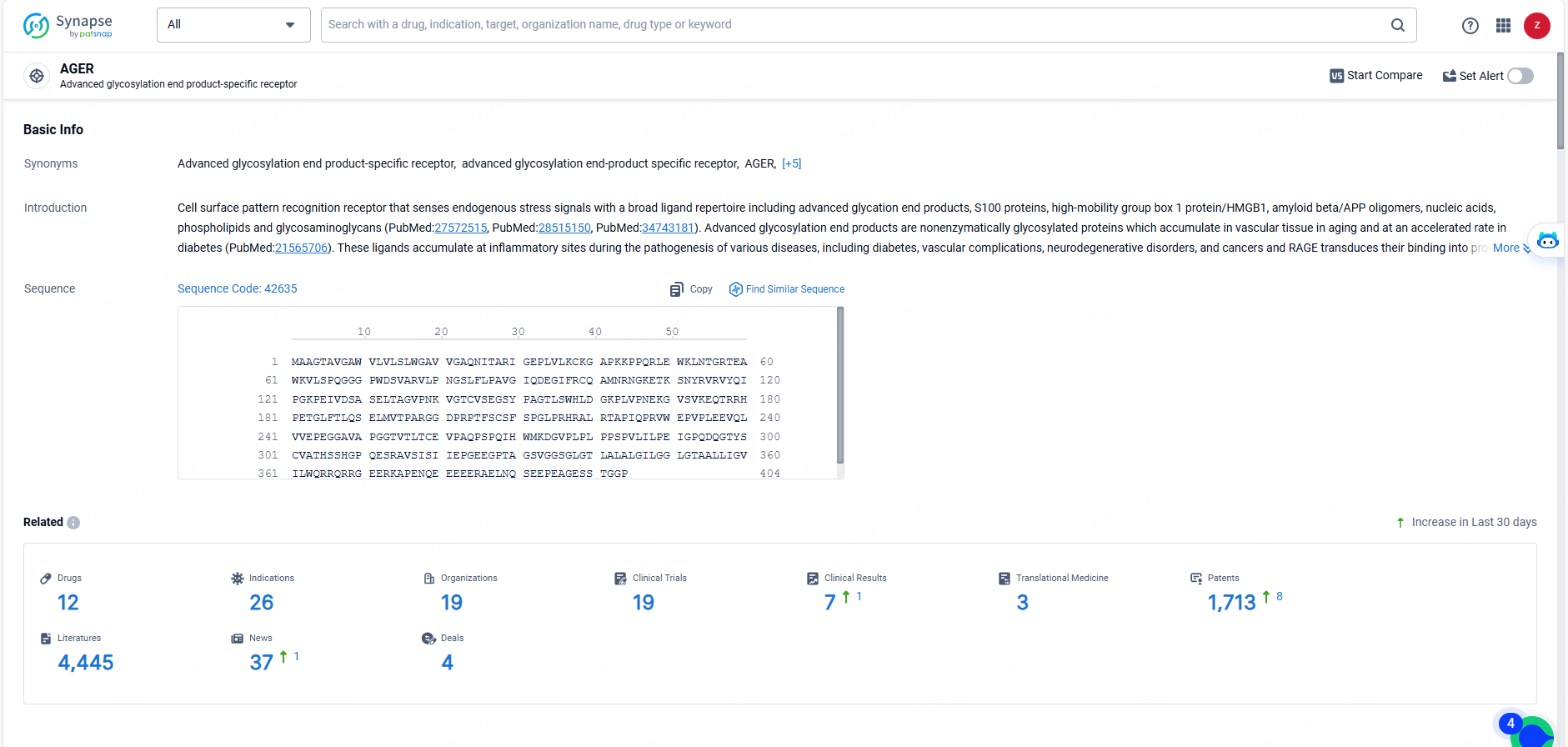
According to the data provided by the Synapse Database, As of May 23, 2024, there are 12 investigational drugs for the AGER target, including 26 indications, 19 R&D institutions involved, with related clinical trials reaching 19, and as many as 1713 patents.
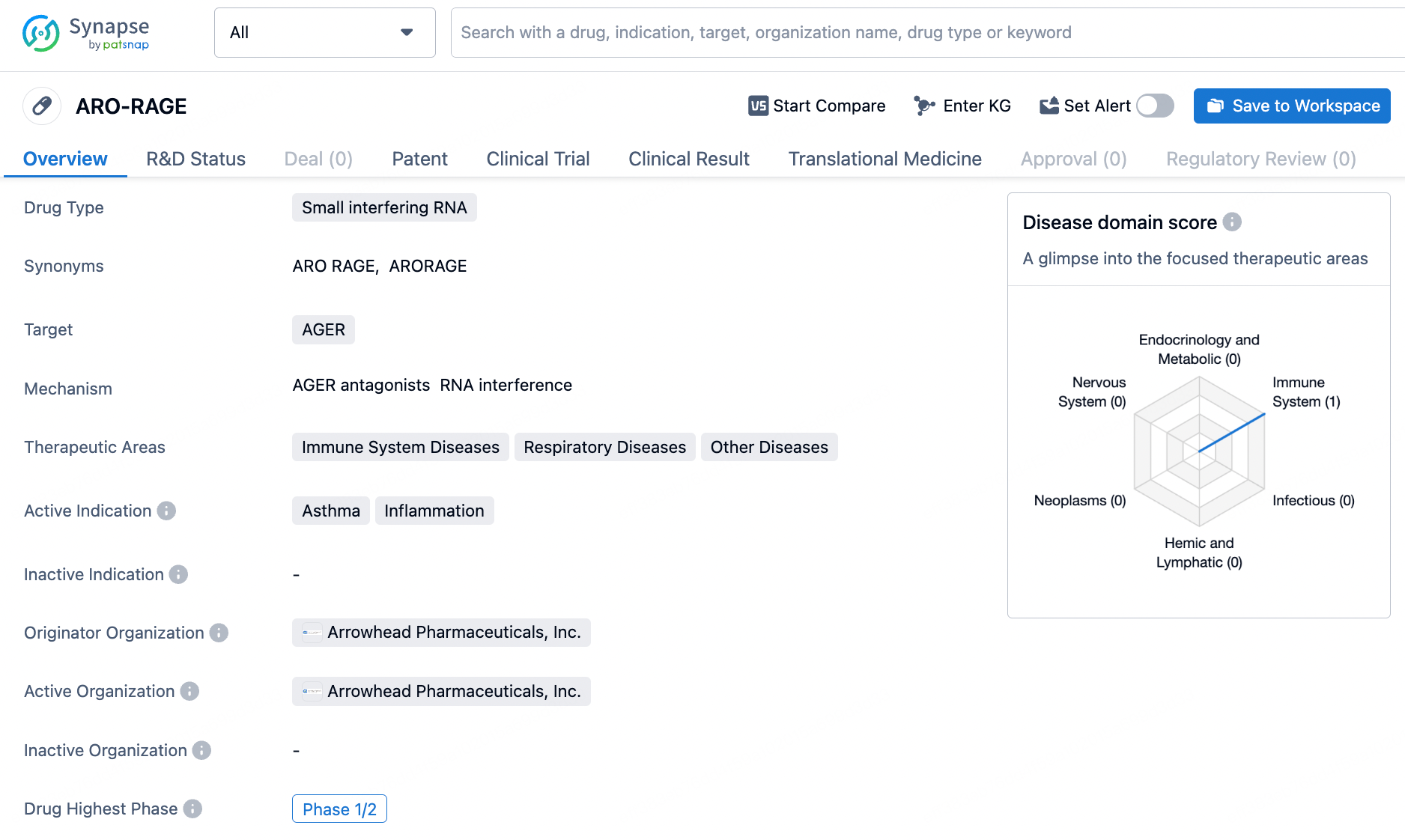
ARO-RAGE targets AGER and is being developed for the treatment of immune system diseases, respiratory diseases, and other diseases. With active indications for asthma and inflammation, ARO-RAGE has reached Phase 1/2 of development, indicating its progress through early clinical testing. The drug's potential to modulate immune and inflammatory pathways suggests its promise in addressing a range of medical conditions related to immune dysregulation.