Astellas files NDA in China for CLDN18.2-targeted new drug Zolbetuximab
On August 1, 2023, the official website of the Chinese Drug Evaluation Center (CDE) shows that the new drug application (NDA) for Zolbetuximab injection developed by Astellas has been accepted for review. The drug is used for the treatment of stomach cancer or gastroesophageal junction (GEJ) adenocarcinoma. This is the first NDA for a CLDN18.2 monoclonal antibody in China. Moreover, Zolbetuximab is currently undergoing NDA review in the United States, Europe, and Japan.
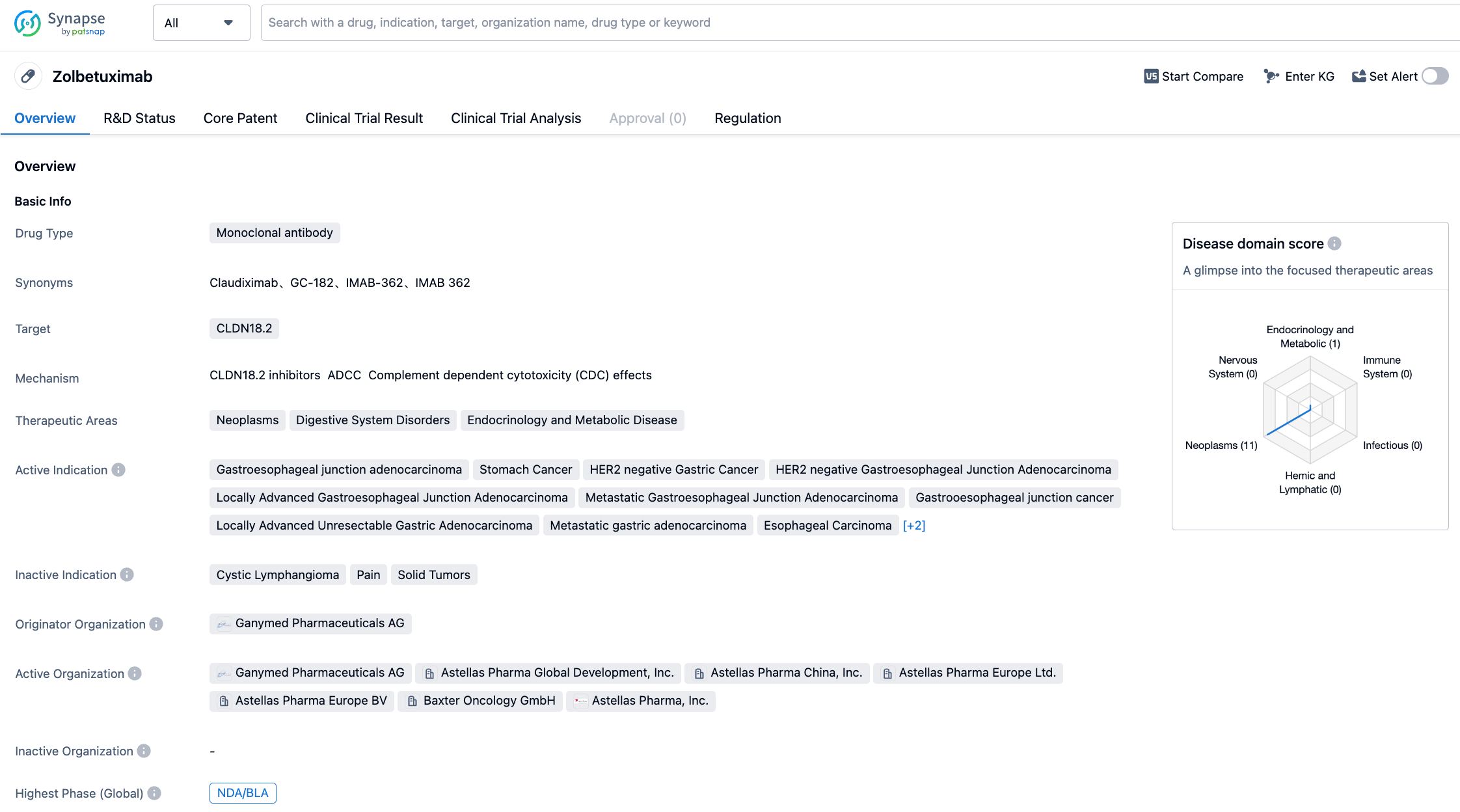
Zolbetuximab is an original chimeric IgG1 monoclonal antibody targeting CLDN18.2, first developed by Ganymed. It binds to CLDN18.2, a transmembrane protein, and performs its function by binding to CLDN18.2 on the surface of stomach cancer cells. In October 2016, Ganymed was acquired by Astellas for 1.28 billion euros, and the core asset of the acquisition is Zolbetuximab. Currently, the Biologics License Application (BLA) for Zolbetuximab as a first-line treatment for locally advanced unresectable or metastatic HER2-negative stomach or GEJ adenocarcinoma positive for Claudin18.2 is undergoing priority review in the United States, with a PDUFA goal date of January 12, 2024. If approved, Zolbetuximab will be the world's first approved drug targeting CLDN18.2. Preclinical research has indicated that after binding, Zolbetuximab activates two different immune system pathways - antibody-dependent cell cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), leading to cancer cell death.
The NDA is mainly based on the results from phase III SPOTLIGHT and GLOW clinical trials. The SPOTLIGHT trial was designed to evaluate the efficacy and safety of Zolbetuximab combined with mFOLFOX6 (a combination regimen including oxaliplatin, folinic acid, and fluorouracil), as a first-line treatment for patients with locally advanced unresectable or metastatic G/GEJ adenocarcinoma who are positive for CLDN18.2 and negative for HER2. The results showed a statistically significant improvement in progression-free survival (PFS) and overall survival (OS) compared to the placebo plus mFOLFOX6 group. In detail, the combination of Zolbetuximab and mFOLFOX6 reduced the risk of disease progression or death by 24.9%, achieving the main study goal. The median PFS in the treatment group was 10.61 months, compared to 8.67 months in the placebo group. The research also demonstrated that Zolbetuximab significantly increased OS in combination with mFOLFOX6 and reduced the risk of death by 25.0%. The median OS for the treatment group and the placebo group were 18.23 months and 15.54 months, respectively.
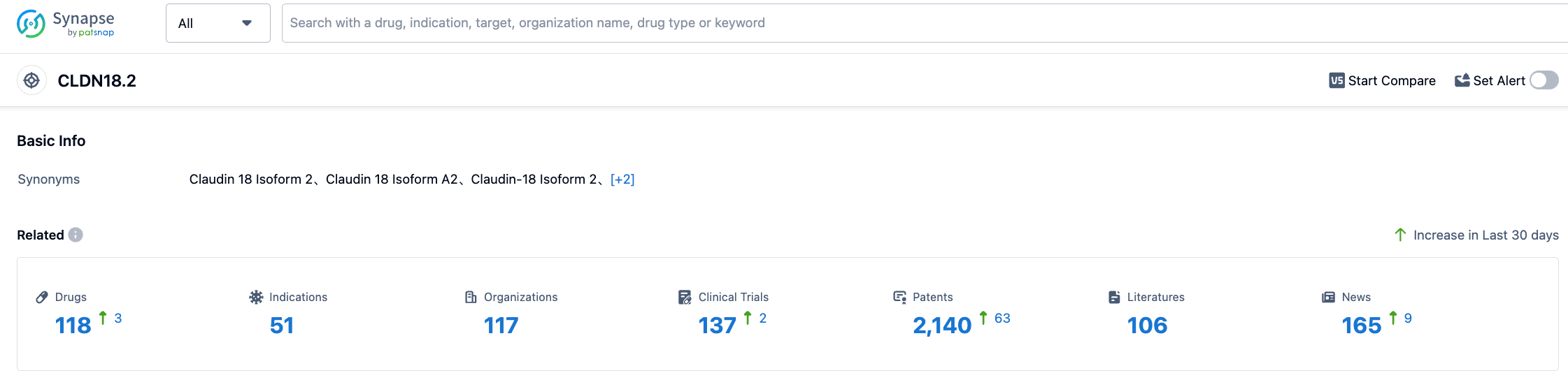
According to information disclosed by Synapse (registered users can freely access detailed information on drugs under development, indications, research institutions, clinical trials, patents, etc. for CLDN18.2 by clicking the card below), as of August 2, 2023, there are 118 drugs under development targeting CLDN18.2, covering 51 indications, with 117 research institutions involved, related clinical trials numbering 137 and as many as 2132 patents. The development of CLDN18.2-targeted drugs is very heated both domestically and internationally. It is expected that domestically developed CLDN18.2-targeted drugs can enter the market soon to compete with foreign new drugs on the same stage.