Progress in the Research and Development of GIPR Drug Targets
Glucose-dependent insulinotropic polypeptide (GIP) is one of two enteroinsular hormones, playing a crucial role in linking nutrient intake with whole-body metabolism. Although GIP is one of the earliest discovered enteroinsular hormones, research on another incretin hormone - glucagon-like peptide 1 (GLP-1) - has far outpaced that on GIP in recent years.
Early research on GIP led to the theory of GIP-induced obesity, curbing interest in GIP receptor agonists in the treatment of type 2 diabetes. However, a resurgence in GIP research has occurred over the past five years, reigniting interest in this peptide. Additionally, two independent approaches to obesity treatment have emerged, namely GIP receptor agonists and antagonists.
There is a plethora of evidence supporting the positive impact of the GIP receptor agonists and antagonists on body weight. The long-standing theory of GIP causing weight gain comes entirely from loss-of-function studies and there is no evidence supporting that GIP receptor agonists lead to obesity or weight gain. Concurrently, there is insufficient evidence to explain the contradictory observation about weight loss with GIP receptor agonists and antagonists. Some hypotheses have pointed to compensatory relations between incretin receptors, and the possibility of GIP receptor antagonist enhancing GLP-1 receptor activity. Moreover, long-term use of GIP receptor agonists may lead to tolerance and the eventual loss of antagonist effect on GIP receptor activity.
Competitive Landscape
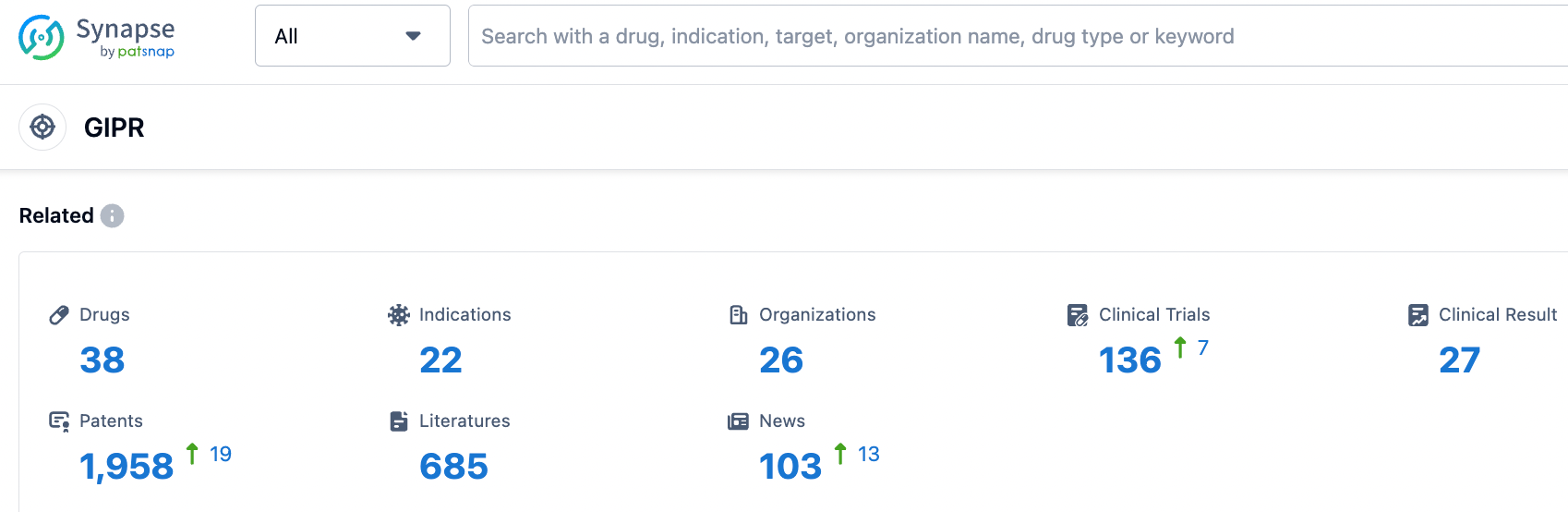
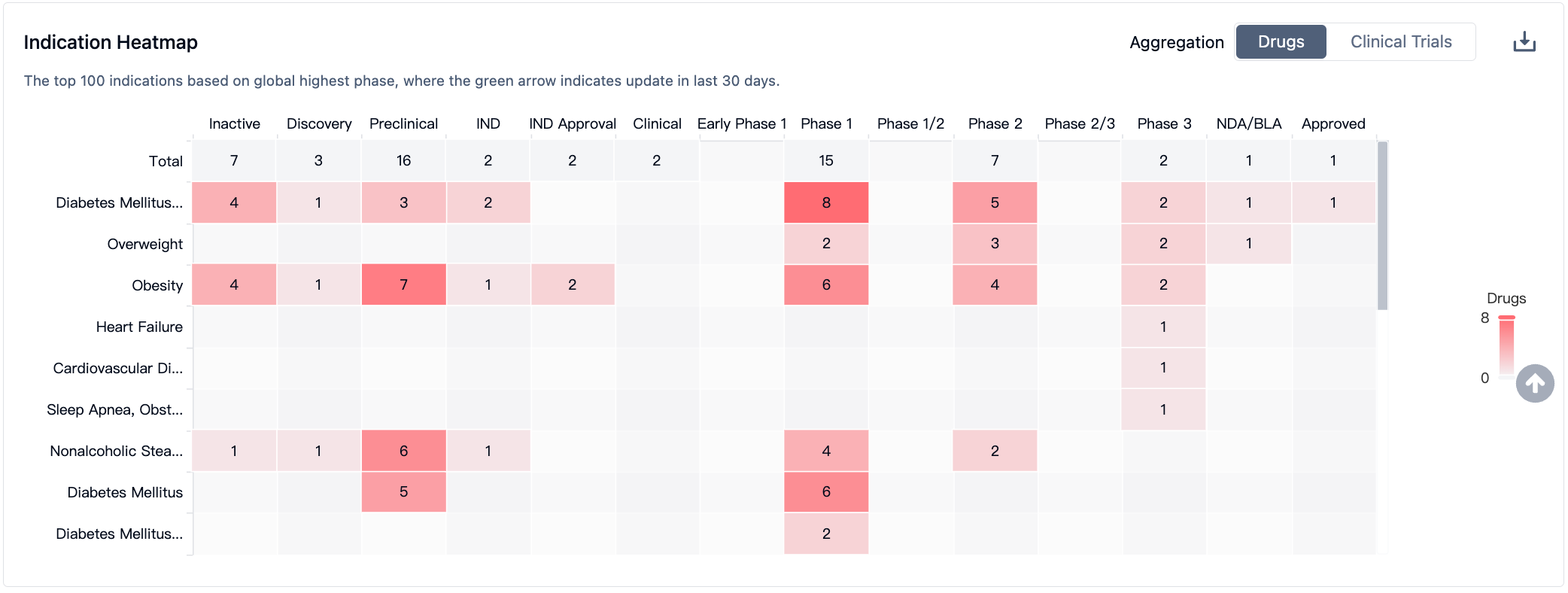
According to information from the Synapse database, nearly 38 varieties are currently in development (including multi-target ones), distributed across various preclinical and clinical stages. These under-development varieties cover nearly 22 diseases, with a primary focus on Type 2 diabetes. The number of patents associated with this target is approximately 1958 (considering multiple targets). From these data, it can be seen that the current value of the GIPR target is mainly concentrated on driving the renewal of GLP1 agonists in the field of diabetes, in order to avoid nausea and vomiting caused by treatment with GLP1 class drugs.
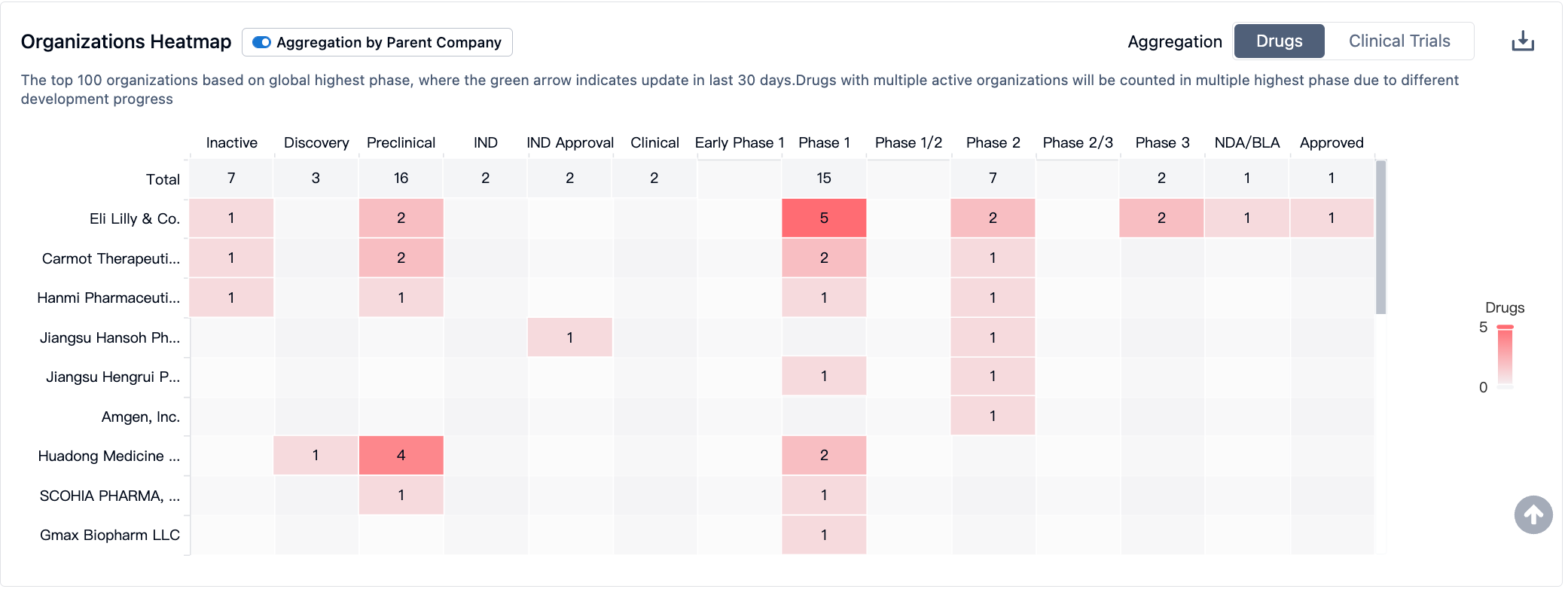
In the field of GLP-1R/GIPR, Lilly's Trelagliptin leads the pack and has been approved by the FDA for marketing. Meanwhile, GLP-1R/GIPR/GCGR triple-target agonists may demonstrate stronger therapeutic effects; Lilly and Hanmi Pharmaceutical have advanced their respective products to phase II clinical trials. As for single-target GIPR drugs, they are still in phase I clinical trials.
Key Drug: Tirzepatide
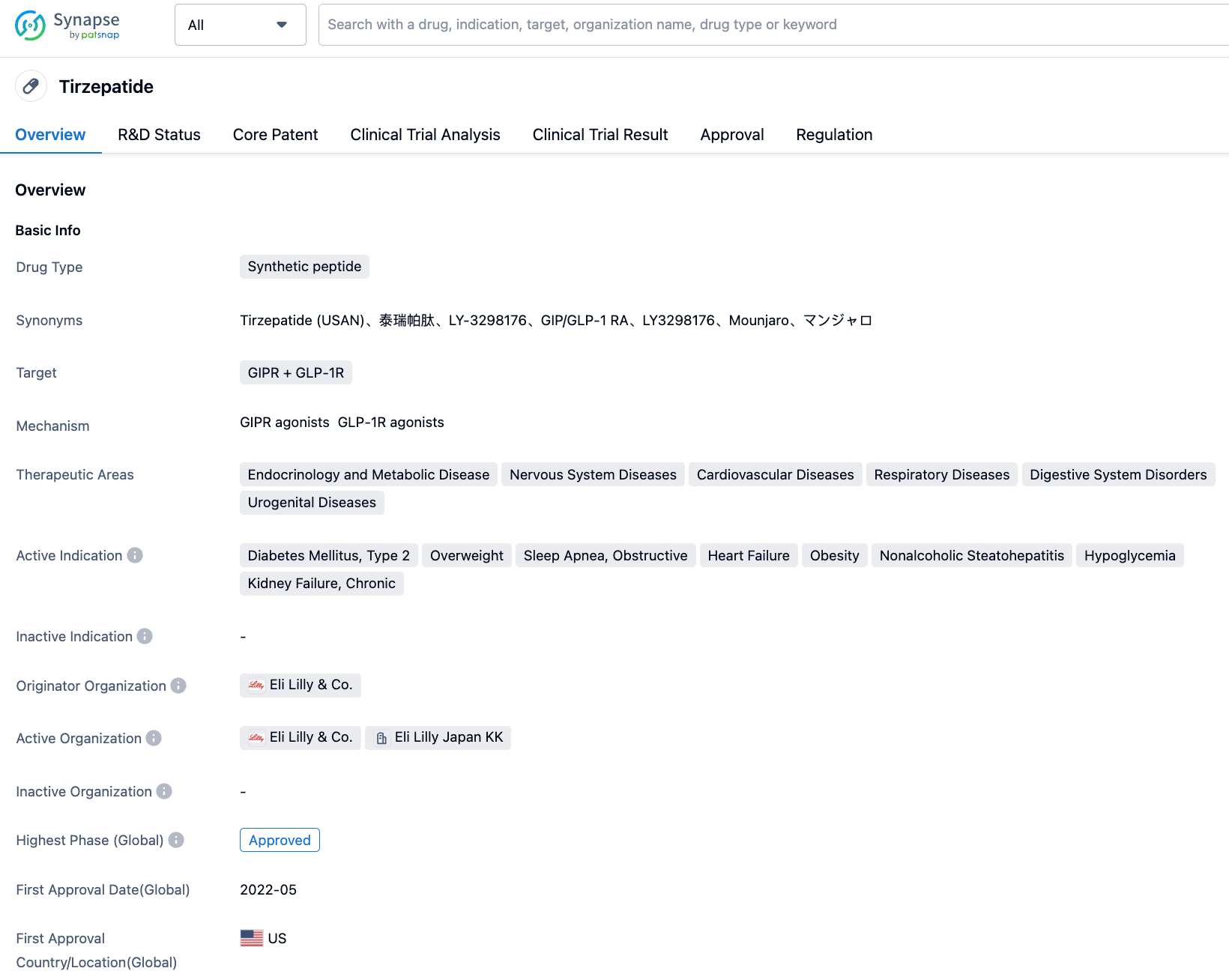
Tirzepatide is a first-in-class dual agonist for the glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) receptors, developed by Eli Lilly. It received FDA approval in May 2022 for improving blood glucose control in adult patients with type 2 diabetes. This medication is administered via subcutaneous injection once a week and can improve blood glucose control through its dual-action mechanism.
Tirzepatide is a linear peptide comprised of 39 amino acids, which binds to the C20 diacid group of fatty acids via a linker conjugated with 20 lysine residues. The peptide sequence of Tirzepatide also contains two non-coding amino acid residues at positions 2 and 13, and the C-terminal of the sequence has been amideified. This amidation technology allows Tirzepatide to bind to albumin, playing a critical role in making possible the delivery regimen of once a week for patients. The molecular weight of Tirzepatide is 4810.52 Da.
As the first novel diabetes therapy type introduced in the past decade, Tirzepatide displays excellent clinical data not only as a monotherapy but also in combination with other drugs. Furthermore, in the phase III SURPASS-2 clinical trial, Tirzepatide was pitted head-to-head against Semaglutide. This showed superior glycaemic control and weight reduction compared to Semaglutide.
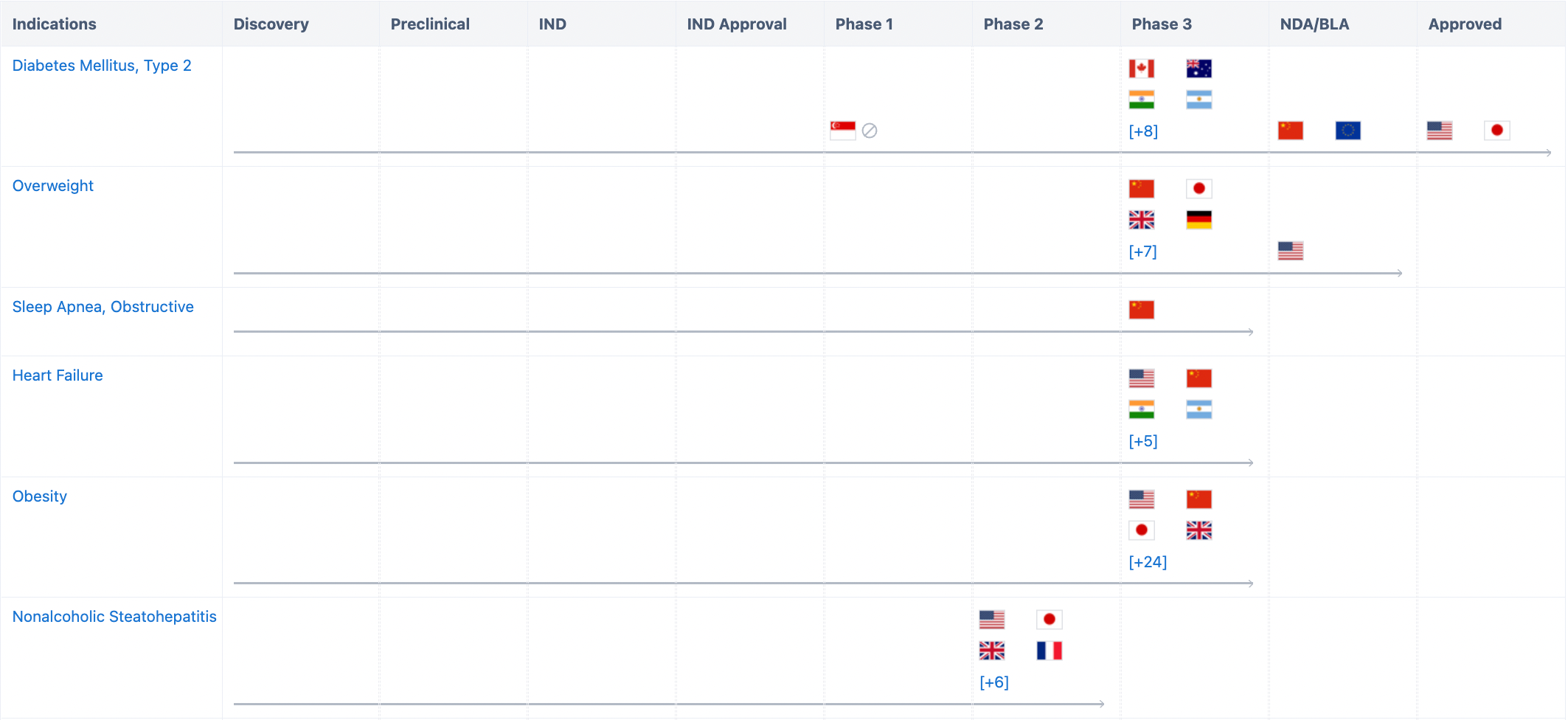
In addition to its approved indications, Tirzepatide is currently conducting several clinical trials worldwide for obstructive sleep apnea and obesity, overweight/obesity, reducing risk of cardiovascular disease in type 2 diabetes, and non-alcoholic steatohepatitis (NASH), etc.
In China, Tirzepatide has also conducted various clinical trials, including those for indications such as Type 2 diabetes, overweight/obesity, and obstructive sleep apnea syndrome. It officially submitted a marketing authorization application to CDE on September 7, 2022, for the treatment of adult patients with type 2 diabetes.