Progress in the Research and Development of JAK3 Drug Targets
Janus kinase is a family of proteins that play a significant role in immune responses by transmitting signals from over 50 different cytokines, thus potentially serving as a target for treating autoimmune diseases.
However, available JAK inhibitors currently lack specificity in inhibiting cytokine signaling, leading to some adverse reactions. Therefore, the next-generation inhibitors need to maintain their efficacy while avoiding adverse events. Within the JAK family, JAK3 is a narrow-spectrum member that only regulates γc cytokines, thus becoming an ideal potential target. The Janus kinase family has four subtypes: JAK1, JAK2, JAK3, and TYK2 (Tyrosine Kinase 2), which transmit signals through the JAK/STAT pathway. JAK1, JAK2, and TYK2 are widely expressed, while JAK3 is mainly expressed in lymphatic tissues and is a selective regulator of lymphocyte development, playing a crucial role in the immune system. JAK3 selectively binds to a cytokine receptor subunit (common γ-subunit or γc chain).
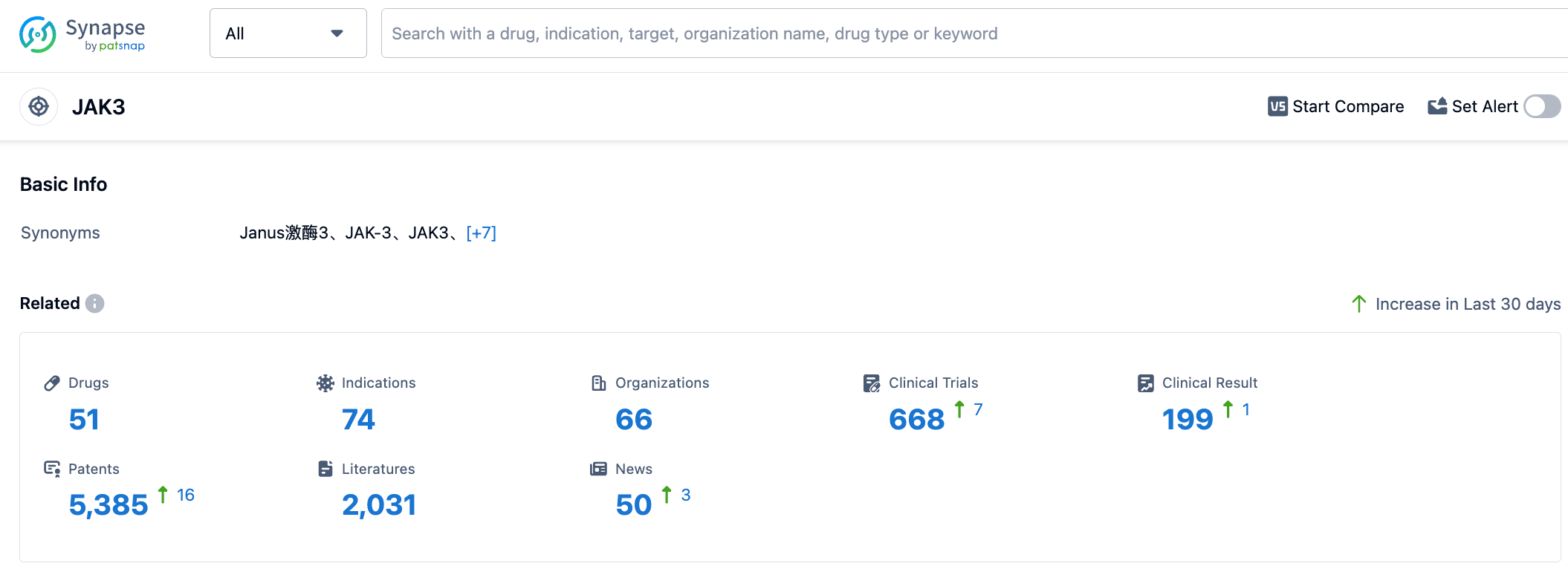
To date, 51 drugs targeting JAK3 have been discovered, covering 74 different indications. The number of associated research and development organizations has reached 66. Clinical trial data shows that there are 199 clinical trial results related to JAK3 targeted drugs. Furthermore, research and development of JAK3 targeted drugs have received widespread attention and protection, with currently 5,385 patent applications related to JAK3 targeted drugs. To support the progress of JAK3 targeted drug research, there have been 2,031 published papers, providing essential references for further research.
Competitive Landscape
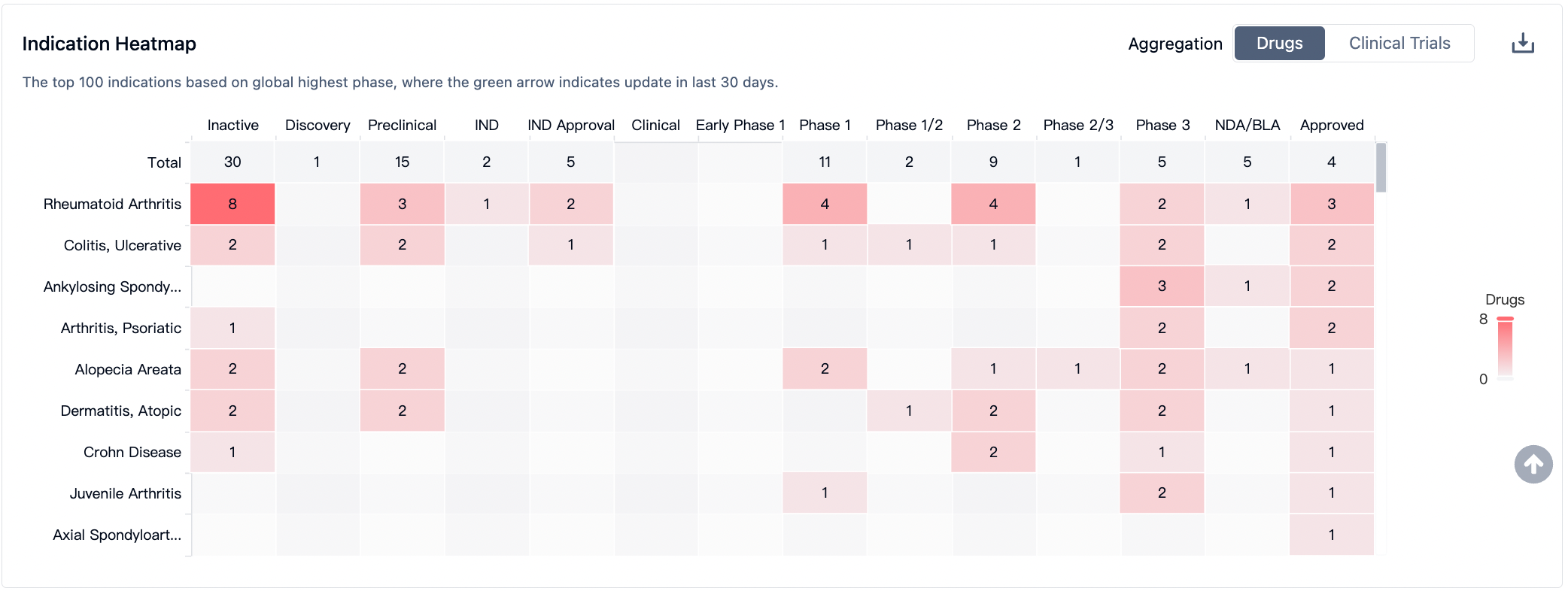
According to the Synapse Database, drugs targeting the JAK3 are being extensively developed, covering multiple therapeutic areas, with a particular focus on autoimmune diseases. For rheumatoid arthritis, there are currently 20 drugs in development, and for ulcerative colitis and atopic dermatitis, there are 10 and 8 drugs respectively under development.
Key Drug
Ritlecitinib is an irreversible inhibitor targeting JAK3. Pfizer plans to conduct Phase II clinical trials (NCT05743244) for type 1 diabetes in 2023, making Ritlecitinib one of the fastest progressing JAK3 selective inhibitors currently.
Ritlecitinib exerts its inhibitory activity by covalently binding with the Cys909 residue in JAK3, enhancing its selectivity for JAK3. Its IC50 for JAK3 is 33.1 nM, whereas it barely has any inhibitory activity for JAK1, JAK2, and TYK2 (IC50 >10,000 nM). Furthermore, Ritlecitinib also functions by inhibiting Th1 and Th17, achieved by suppressing the production of IFNγ (IC50 = 48 nM) and IL-17 (IC50 = 269 nM).
The mechanism of JAK inhibitors in the treatment of Inflammatory Bowel Disease (IBD) is by blocking the communication between immune cells. In May 2018, the FDA approved the oral small molecule inhibitor Tofacitinib for the treatment of moderate to severe ulcerative colitis. Tofacitinib can inhibit JAK1 and JAK3, thus blocking the downstream JAK/STAT signaling pathway and regulating DNA transcription. The selective JAK1 inhibitor Itacitinib showed good efficacy in patients with acute graft versus host disease (aGVHD), but research on its indications for pancreatic cancer, plaque psoriasis, and rheumatoid arthritis was discontinued due to side effects.
Whether selective JAK3 inhibitors can reduce the side effects of widespread cytokine suppression has at least been confirmed by phase I clinical data from Ritlecitinib. Moreover, no significant adverse cardiac events (MACE), deaths, or opportunistic infections were observed in the study.
In September last year, regulatory authorities in the United States and Japan respectively approved the TYK2 inhibitor deucravacitinib for the treatment of moderate to severe plaque psoriasis. Deucravacitinib also became the first selective inhibitor to be marketed in the TYK2 signaling pathway. This successful market case again demonstrates the potential for drug development in the JAK/STAT pathway.
After 30 years of research, the understanding of this pathway has deepened, and research on the function of JAK kinase subtypes has gradually deepened, and the marketed drugs have developed from early non-selective inhibitors to inhibitors with high selectivity for two subtypes or a certain subtype. Whether a JAK3 highly selective inhibitor can be successfully marketed like the TYK2 inhibitor still needs time to verify.