Zai Lab's new drug Maqituxi Monoclonal Antibody Injection targeting HER2 has been approved in China
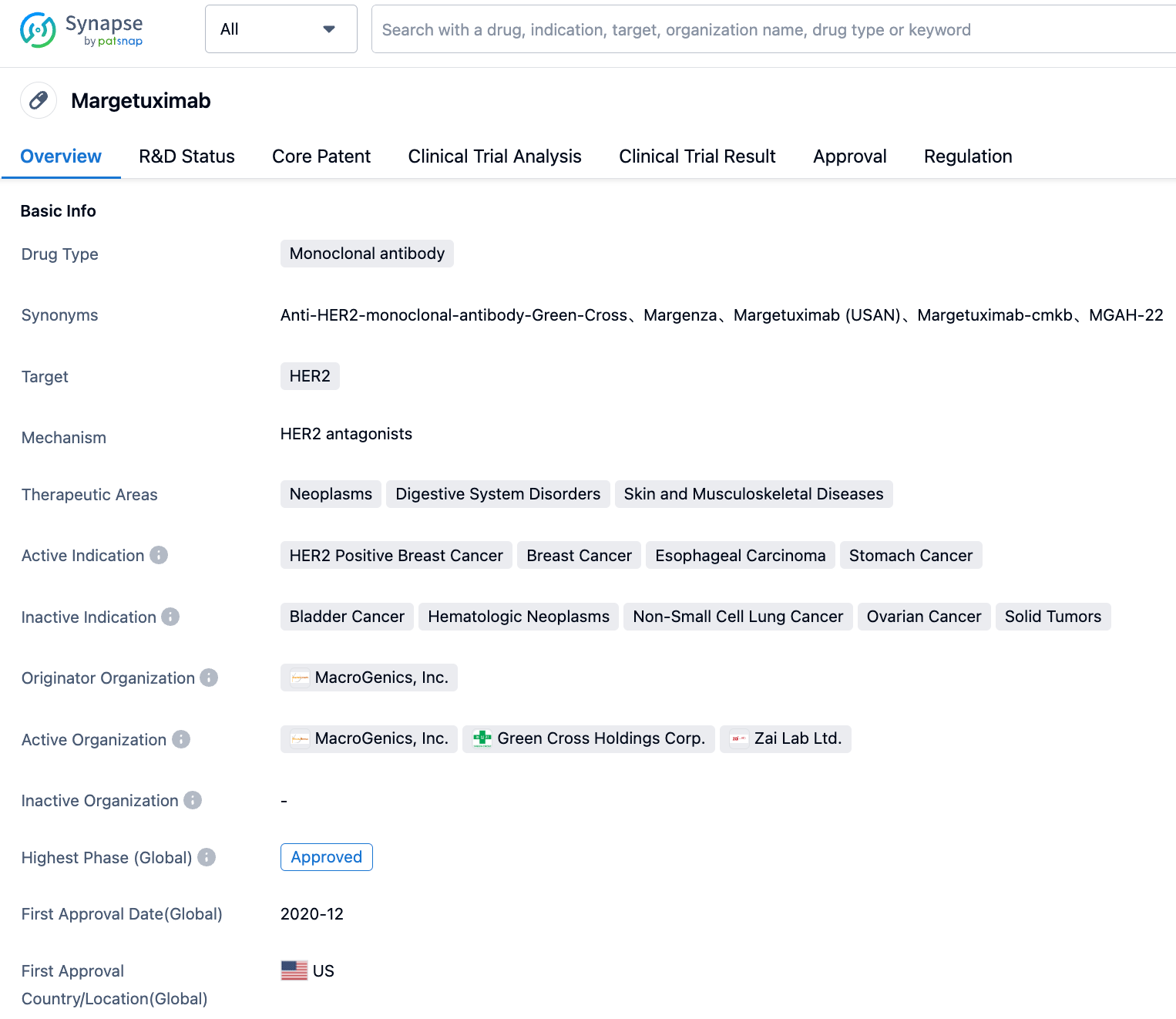
On September 1, 2023, the latest announcement on the official website of the NMPA indicates that the Margetuximab injection, applied for by Zai Lab, has been approved for marketing in China. Public information shows that Margetuximab is an Fc-optimized monoclonal antibody acting on HER2, which was introduced by Zai Lab from MacroGenics. The approved indications are for third-line and above treatment of patients with HER2-positive metastatic breast cancer.
Margetuximab, originally researched by MacroGenics, is an Fc-optimized monoclonal antibody targeting Human Epidermal Growth Factor Receptor 2 (HER2). It enhances binding and activation with stimulatory FcγR CD16A and reduces binding with inhibitory FcγR CD32B. This allows it to bind more tightly to effector cells and enhance ADCC effects while retaining anti-tumor proliferative characteristics similar to Trastuzumab. In November 2018, Zai Lab reached a $165 million collaboration with MacroGenics, gaining exclusive rights to develop and commercialize three immunooncology drugs in the Greater China region (including mainland China, Hong Kong, Macau, and Taiwan), one of which is Margetuximab. In vitro data show the optimized Fc domain increases affinity for the activating Fc receptor FCGR3A (CD16A) and decreases affinity for the inhibitory Fc receptor FCGR2B (CD32B). These changes enhance ADCC effects and NK cell activation.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The launch is based on positive data from the global Phase III SOPHIA study and registration bridging study. The SOPHIA study results show that margetuximab is the only anti-HER2 treatment that has demonstrated improved PFS in head-to-head studies with trastuzumab, highlighting the potential of margetuximab as a critical new treatment option for refractory patients.
Compared with trastuzumab combined with chemotherapy, margetuximab combined with chemotherapy significantly reduced the risk of disease progression or death in patients, with statistical significance (HR=0.76). The median progression-free survival (mPFS) of the two groups was 5.8 months and 4.9 months, respectively. In terms of the objective remission rate, the rate in the margetuximab combined with chemotherapy group was 22%, higher than the 16% in the control group. Based on this research, the U.S. FDA approved margetuximab for marketing in 2020.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
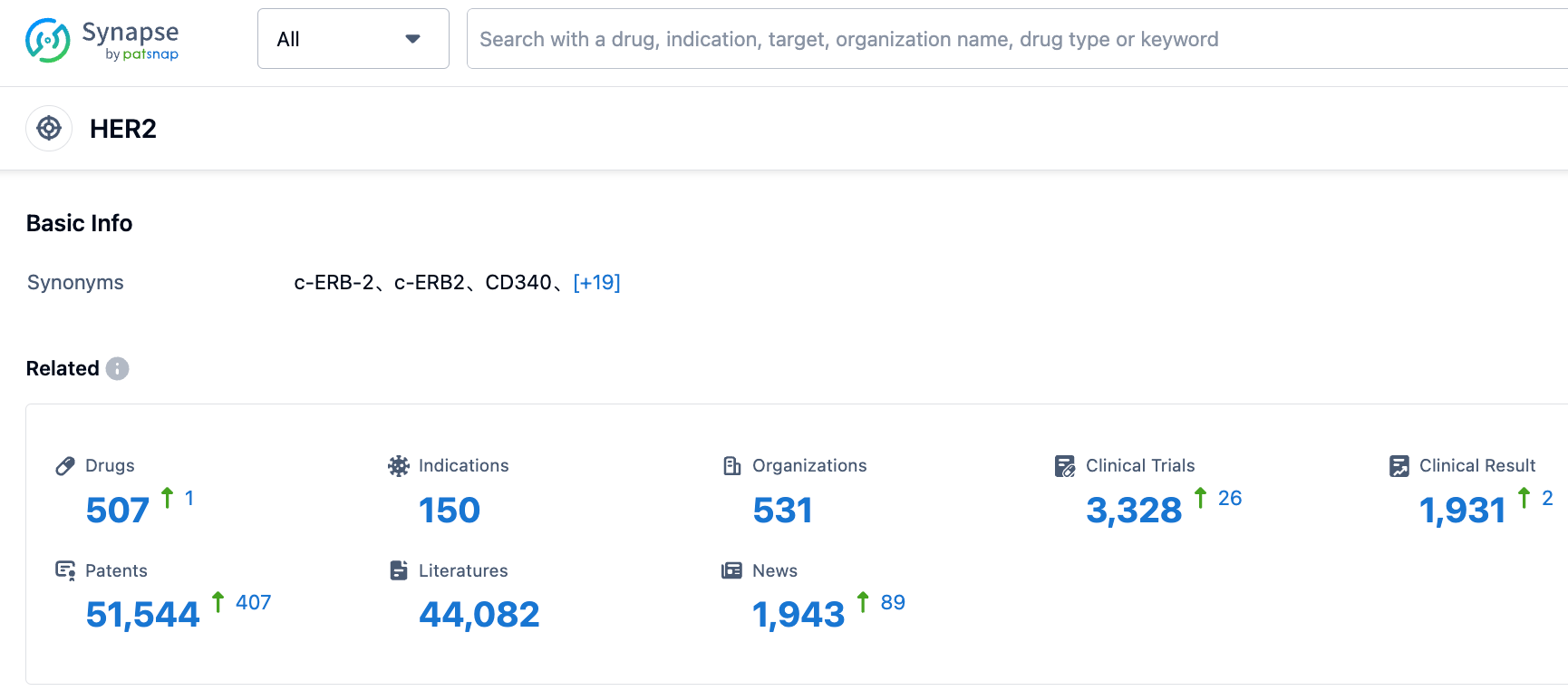
According to the information disclosed by the Synapse database, as of September 2, 2023, there are a total of 507 drugs under investigation targeting HER2, including 150 different indications, involving 531 research institutions, related to 3,317 clinical trials, and as many as 51,590 patents.
In breast cancer patients, many overexpress the human epidermal growth factor receptor 2 (HER2). The HER2 protein promotes the growth of cancer cells, severely affecting the treatment and prognosis of patients; more than 25% of women with metastatic HER2-positive breast cancer will develop brain metastasis. The launch of Margetuximab in China offers a new treatment option for a large number of breast cancer patients.