Sirnaomics announced positive results from phase 1 clinical trials of siRNA therapy STP707
On August 31, 2023, Sirnaomics announced positive results from Phase 1 clinical trials of its RNAi therapy STP707 for treating various solid tumors. The trial targeted patients suffering from various late-stage solid tumors and unresponsive to standard treatments, with all dosing cohorts now completed. The latest data revealed that about 74% of evaluable patients presented the best response of stable disease (SD), with several patients judged to have reduced tumor load according to the criteria for evaluating the effect of solid tumors.
STP707 is composed of two siRNA oligonucleotides targeting TGF-β1 and COX-2 mRNA, combined and formulated with the carrier of Histidine-Lysine Co-polymer (HKP+H) into nanoparticle preparations. Each individual siRNA possesses the capability of inhibiting the expression of their corresponding target mRNAs. STP707 is also able to simultaneously inhibit the expression of TGF-β1 and COX-2, thereby producing a synergistic effect and reducing inflammations.
Overexpression of TGF-β1 and COX-2 has been proven to play a key regulatory role in tumor formation. STP707 is designed to leverage its dual-target inhibition properties and Sirnaomics' unique Polymeric Nanoparticle (PNP) introduction technology. By systemic administration, it improves the drug's targeted delivery to solid tumors and metastatic tumors. In a preclinical study of STP707, knockdown of TGF-β1 and COX-2 gene expression was observable in organs including the liver and lungs following intravenous injection. Furthermore, in various preclinical models, STP707 exhibited anti-tumor activity against several types of solid tumors.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The positive results were from a multicenter, open-label, dose escalation, and dose-extension Phase 1 clinical study, carried out by several oncology clinical institutions in the United States. The study examined the safety, tolerability, and antitumor activity of STP707 when administered intravenously. A total of 50 patients with late-stage solid tumors were recruited, including but not limited to pancreatic cancer, liver cancer, colon cancer, ovarian cancer, and melanoma, all of whom had continued to show progressive disease after receiving marketed cancer treatment drugs. This clinical trial was a multicenter, open-label, dose-escalating study evaluating the safety, tolerability, and antitumor activity of STP707. The 50 subjects received doses of 3 mg, 6 mg, 12 mg, 24 mg, 36 mg, and 48 mg via intravenous administration. All patients received one dose per week, with a total of four doses in a 28-day treatment cycle.
The secondary endpoints of the trial were to determine the pharmacokinetics, biomarkers, and observe preliminary anti-tumor activity of STP707. Based on preliminary efficacy observations, 74% of evaluated subjects achieved the best response of stable disease, and a few patients saw a reduction in their tumor load.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
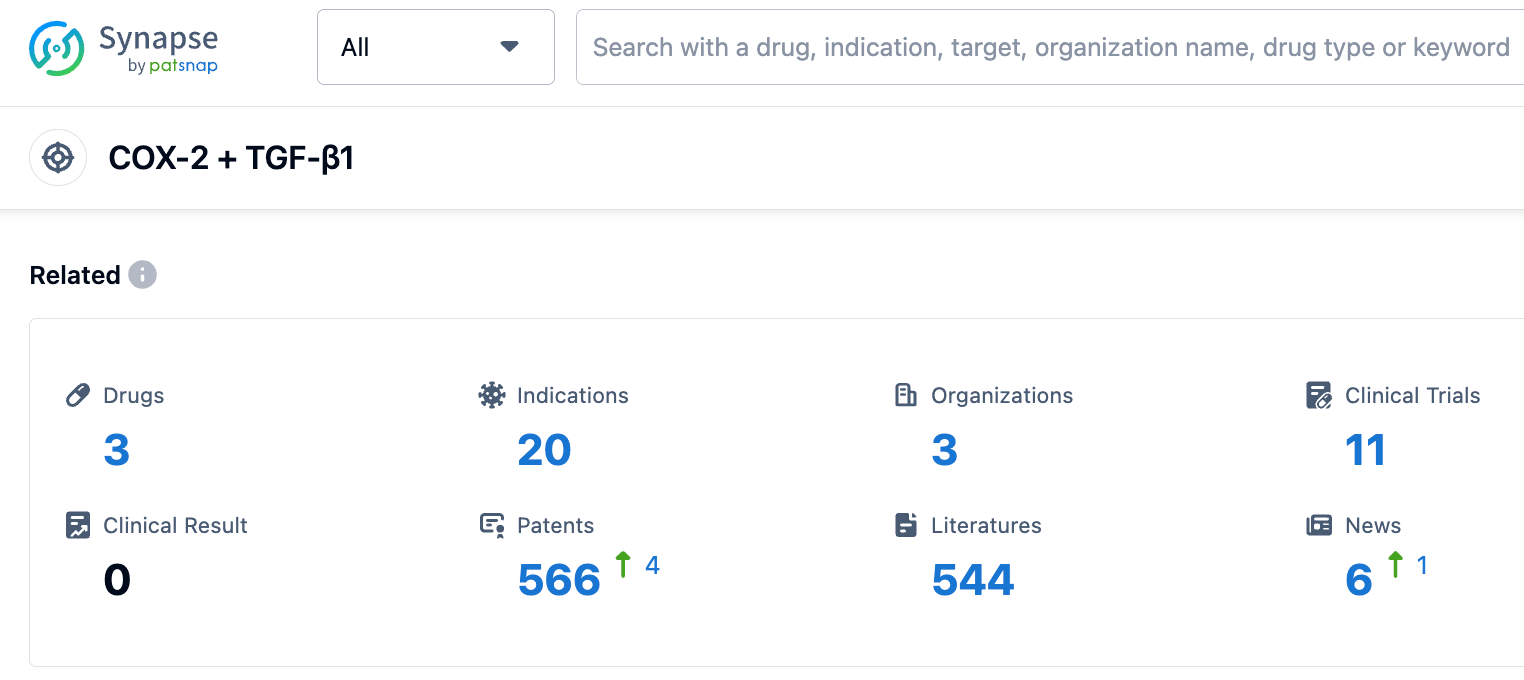
According to information disclosed by the synapse database, as of September 1, 2023, there are three drugs under research for COX-2 + TGF-β1 targets, covering 20 indications, being studied by three institutions, involving 11 related clinical trials, and up to 566 patents.
Based on the positive mid-term data of STP707, Sirnaomics plans to explore collaboration for Phase II combination trials, combining STP707 with newly approved cancer therapies (such as immune checkpoint inhibitors) as well as traditional chemotherapy. Potential combination therapies may include cholangiocarcinoma (CCA), hepatocellular carcinoma (HCC), melanoma, or pancreatic cancer. The RNAi treatment demonstrates massive potential in treating a variety of diseases, including cancer, diabetes, hepatitis B and specific cardiovascular disorders, looking forward to the performance of STP707 in the field of oncology.