EyeDNA Therapeutics Presents Promising 2-Year Phase 1/2 Study Results on HORA-PDE6b Therapy for Retinitis Pigmentosa at ARVO
eyeDNA Therapeutics, which is a recent spin-off from Coave Therapeutics, a firm specializing in genetic medicine aimed at pioneering transformative treatments, revealed encouraging results from the 24-month follow-up of their Phase I/II clinical trial. This study assessed the safety and effectiveness of HORA-PDE6b, the company’s experimental gene therapy designed for the treatment of retinitis pigmentosa resulting from bi-allelic mutations in the PDE6b gene.
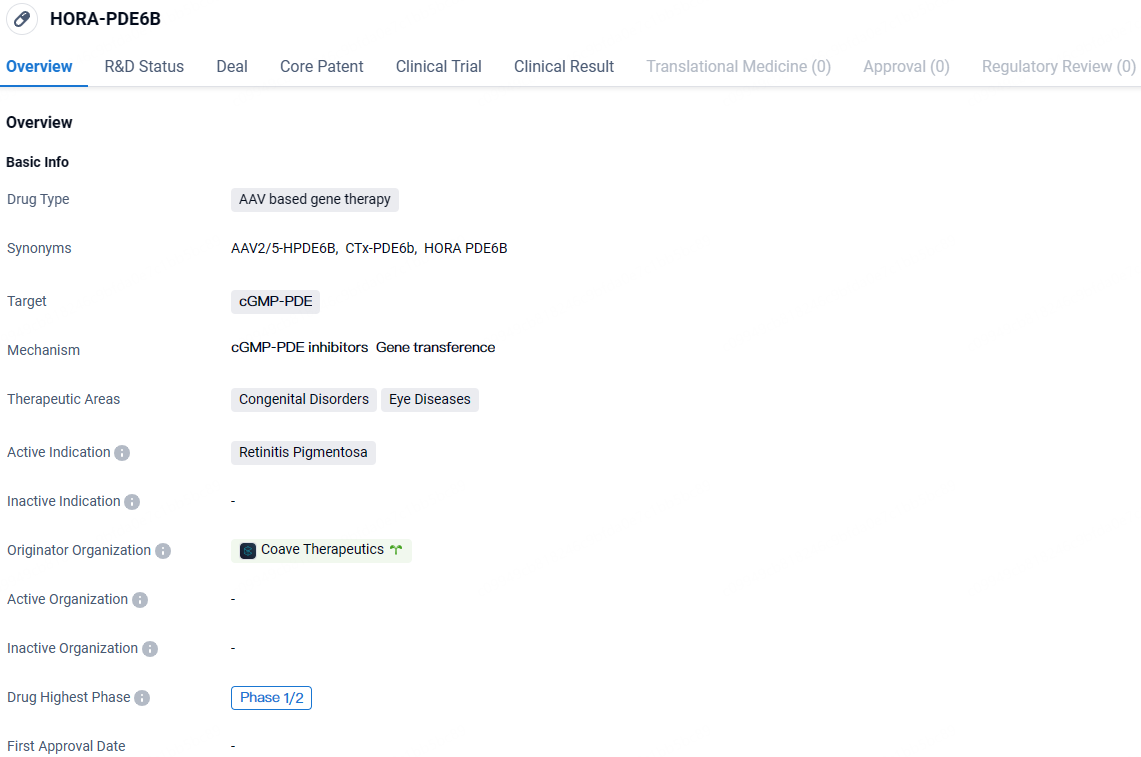
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The 24-month continuation data for HORA-PDE6b reinforces the findings of an earlier interim analysis carried out at 12 months, endorsing the process to initiate a registrational study for those affected with PDE6b RP. We aim to engage with health regulatory bodies in both the US and Europe to chart the most effective course for delivering HORA-PDE6b to patients requiring treatment.
As of now, the administration of HORA-PDE6b in individuals with PDE6b RP has incorporated 17 patients, all 18 years or older, bearing a severe form of the disease. The application involved two incremental doses across four distinct groups. The application targeted the more severely impacted eye, utilizing the other as a control.
Rodolphe Clerval, the CEO, commented, “The encouraging two-year safety profile and effectiveness observed following HORA-PDE6b treatments bolster our belief in its potential as a transformative gene therapy to significantly aid PDE6b RP patients. These findings will guide our forthcoming engagements with regulatory authorities to secure an efficient approval pathway for HORA-PDE6b.”
Dr. Jean-Baptiste Ducloyer, MD, at the Department of Ophthalmology, Nantes University, remarked, “PDE6b retinitis pigmentosa, a relentlessly progressing and incurable genetic disorder, culminates in substantial visual decline and eventual blindness. The promising two-year safety and efficacy results hold substantial clinical value and herald a potentially vital advancement in treating this crippling disorder.”
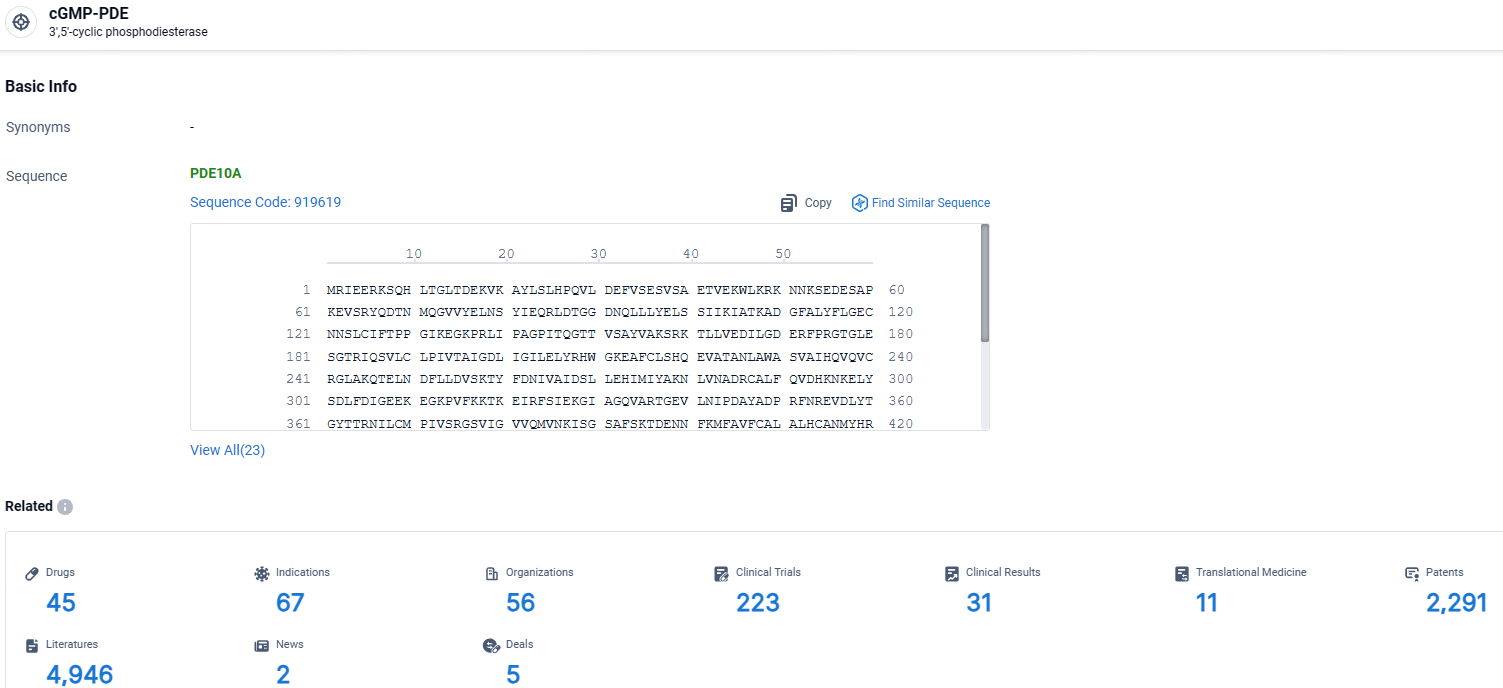
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 9, 2024, there are 45 investigational drugs for the cGMP-PDE targets, including 67 indications, 56 R&D institutions involved, with related clinical trials reaching 223, and as many as 2291 patents.
HORA-PDE6B targets the cGMP-PDE enzyme and aims to restore visual function in patients with this condition. The drug is currently in Phase 1/2 of clinical development, and further research is needed to evaluate its potential as a treatment option for this rare genetic disorder.