What Does Blinatumomab Do?
Blinatumomab, sold under the brand name Blincyto, is a breakthrough cancer treatment developed by Amgen Inc. It is a CD19-directed monoclonal antibody specifically designed for the treatment of acute lymphoblastic leukemia (ALL) in adults and children.ALL is a type of blood cancer characterized by the rapid growth of abnormal white blood cells. Blinatumomab is part of a class of drugs known as CD19 monoclonal antibodies, which are a form of targeted cancer therapy. The drug has shown promise in treating patients with B-cell precursor ALL that is in remission with molecular evidence of leukemia or has relapsed or did not respond well to earlier treatments.
Mechanism of Action of Blinatumomab
Blinatumomab works by binding to the CD19 protein present on the surface of B-cell ALL cells. This binding action stimulates the immune system to recognize and destroy the cancer cells. As a monoclonal antibody, blinatumomab is designed to target only specific cells, which may result in less toxicity to healthy cells compared to traditional chemotherapy. The drug's unique mechanism of action has made it a valuable option for patients with certain types of ALL.
How to Use Blinatumomab
Blinatumomab is administered via intravenous infusion, which is typically given in a hospital or clinical setting by healthcare professionals. The treatment cycle involves a continuous IV infusion for 4 weeks (28 days), followed by a 2-week (14 days) break. This constitutes one treatment cycle, which may be repeated depending on the patient's response to the therapy. Before the infusion, patients are given a corticosteroid to help reduce the risk of infusion reactions. It is crucial to follow the healthcare provider's instructions regarding the administration of the drug and to attend regular medical check-ups to monitor progress and side effects.
Side Effects of Blinatumomab
Blinatumomab can cause a range of side effects, some of which can be severe or life-threatening. These include cytokine release syndrome (CRS), which may present with symptoms such as fever, vomiting, tiredness, chills, dizziness, facial swelling, headache, wheezing, low blood pressure, skin rash, and nausea. Neurologic problems can also occur, manifesting as seizures, loss of balance, difficulty speaking, headache, loss of consciousness, and difficulty with facial movements, hearing, vision, swallowing, sleeping, and confusion.
Common side effects may include infections, low platelet count (thrombocytopenia), fever, infusion-related reactions, headache, and low red blood cell count (anemia). Patients should be closely monitored for these side effects, and treatment may need to be adjusted or discontinued if severe reactions occur.
Drug Interactions with Blinatumomab
Other medications may interact with blinatumomab, potentially affecting its efficacy or increasing the risk of side effects. It is essential to inform healthcare providers of all current medications, including prescription and over-the-counter drugs, vitamins, and herbal supplements. Patients should avoid driving or operating heavy machinery during treatment due to the potential for neurological symptoms that could impair cognitive function.
In conclusion, blinatumomab is a targeted therapy offering a new approach to the treatment of specific types of ALL. While it presents a valuable treatment option for patients with limited alternatives, its use requires careful monitoring and management due to the potential for severe side effects. As with any cancer treatment, patients should engage in a thorough discussion with their healthcare team to understand the benefits and risks associated with blinatumomab therapy.
How to obtain the latest development progress of all drugs?
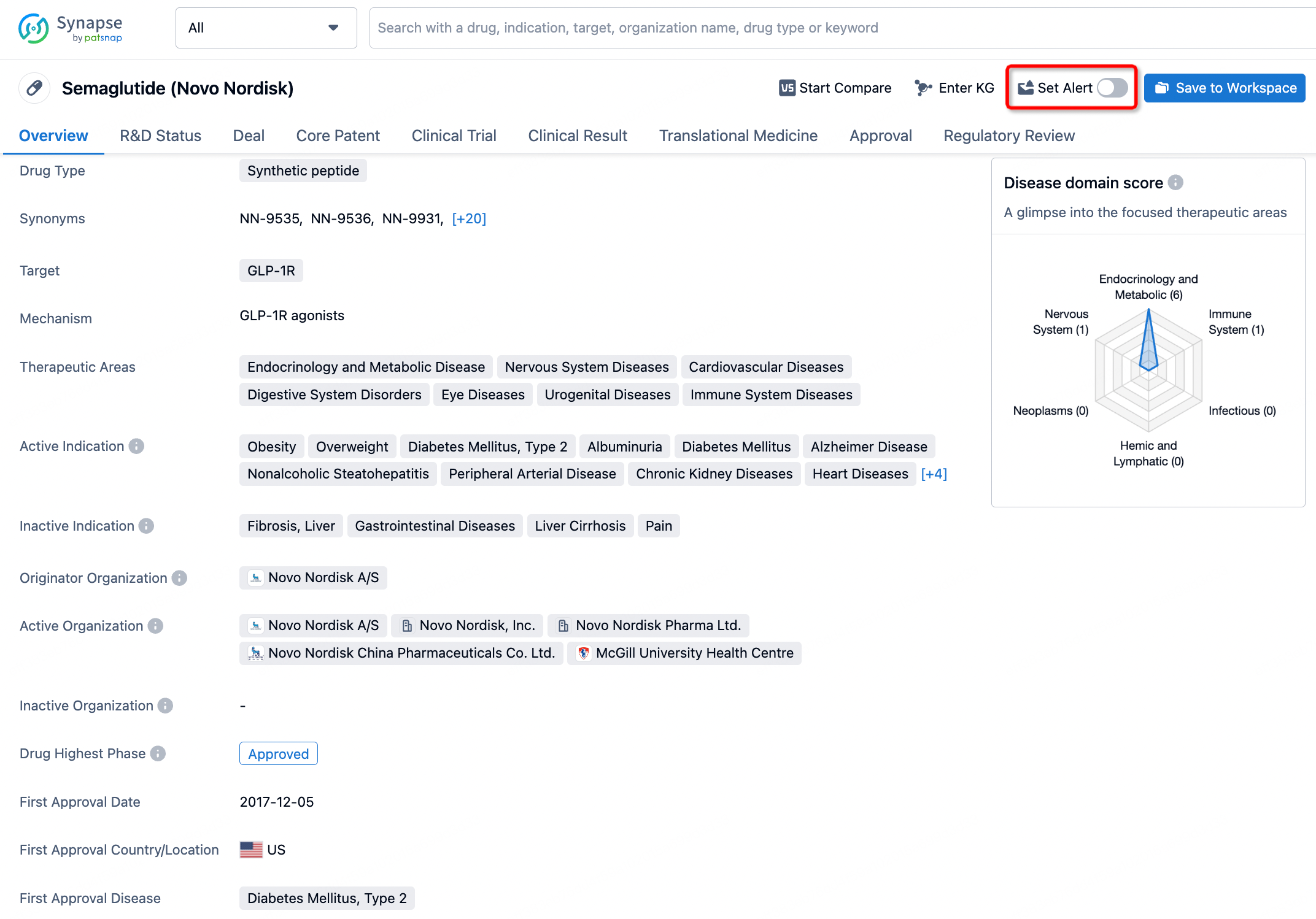
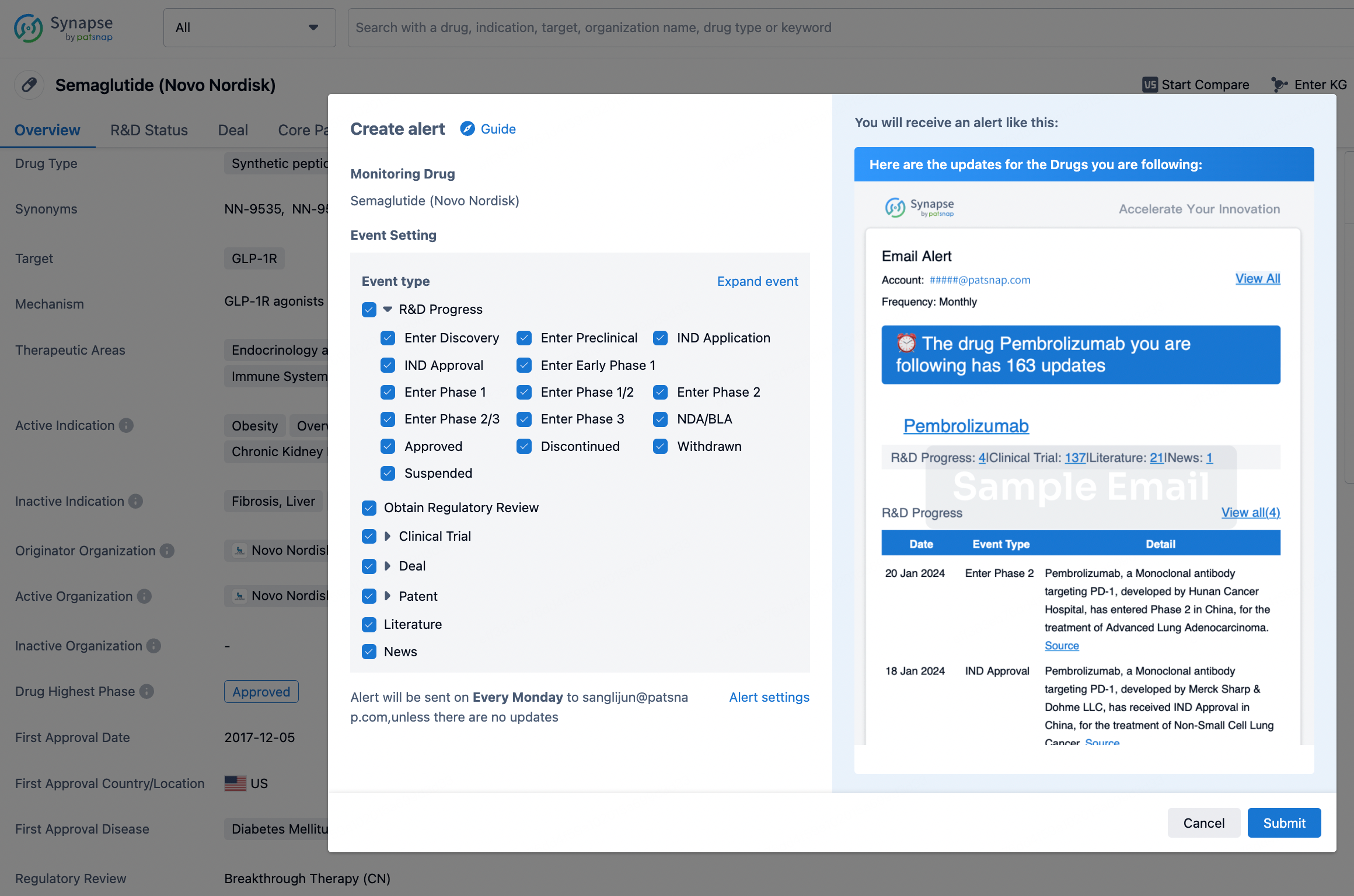
In the Synapse database, you can keep abreast of the latest research and development advances of all drugs anywhere and anytime, daily or weekly, through the "Set Alert" function. Click on the image below to embark on a brand new journey of drug discovery!