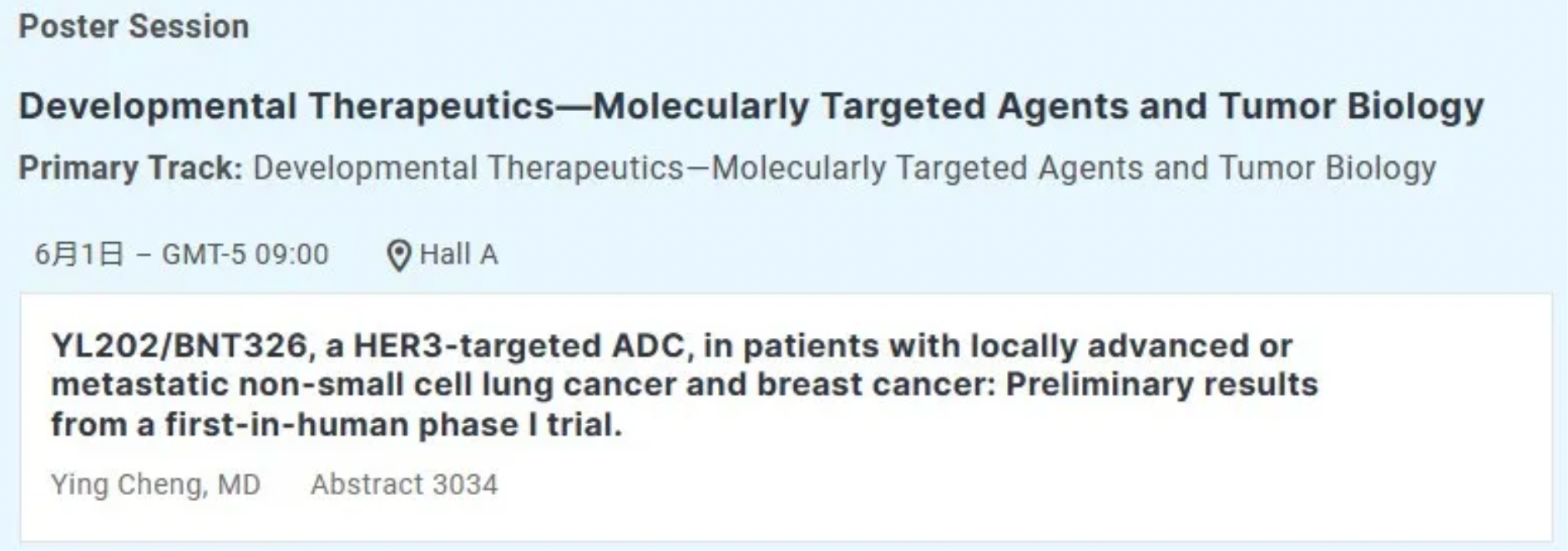
What ADC-related progress will Medilink Therapeutics reveal at the ASCO conference?
Medilink Therapeutics will present preliminary results from the first human clinical Phase 1 trial of YL202 for the treatment of locally advanced or metastatic non-small cell lung cancer and breast cancer (NCT05653752).
In October 2023, Medilink Therapeutics entered into a strategic collaboration and global licensing agreement with BioNTech to develop the next generation of HER3-targeting ADCs. Under the terms of the agreement, Medilink will grant BioNTech the exclusive rights to develop, manufacture, and commercialize YL202 globally, excluding mainland China, Hong Kong, and Macau. BioNTech will pay Medilink an upfront payment of 70 million USD, along with additional payments for development, regulatory, and commercial milestones, with a potential total exceeding one billion USD.
YL202, developed based on the TMALIN technology platform, is the second product from this platform and is expected to provide new evidence for clinical concept validation of the TMALIN technology, offering safer and more effective treatment options for cancer patients worldwide. It is reported that YL202 has successfully completed the first patient dosing in a Phase 1 clinical trial in the United States. This trial is an international multicenter clinical study designed to evaluate the safety, tolerability, pharmacokinetics, and antitumor activity of YL202 in patients with advanced non-small cell lung cancer and breast cancer.
Founded on July 8, 2020, Medilink Therapeutics is a company specializing in the development of innovative drugs focused on ADCs and related technologies. The company has developed the latest generation TMALIN® novel antibody-drug conjugate platform technology with proprietary intellectual property rights. This platform leverages dual cleavage mechanisms both intra- and extracellularly through tumor microenvironments and traditional lysosomes, and features high water solubility, high homogeneity, high stability in vitro and in vivo, and enriched tumor tissue characteristics.
How to search for and analyze the development progress of ADC pharmaceuticals?
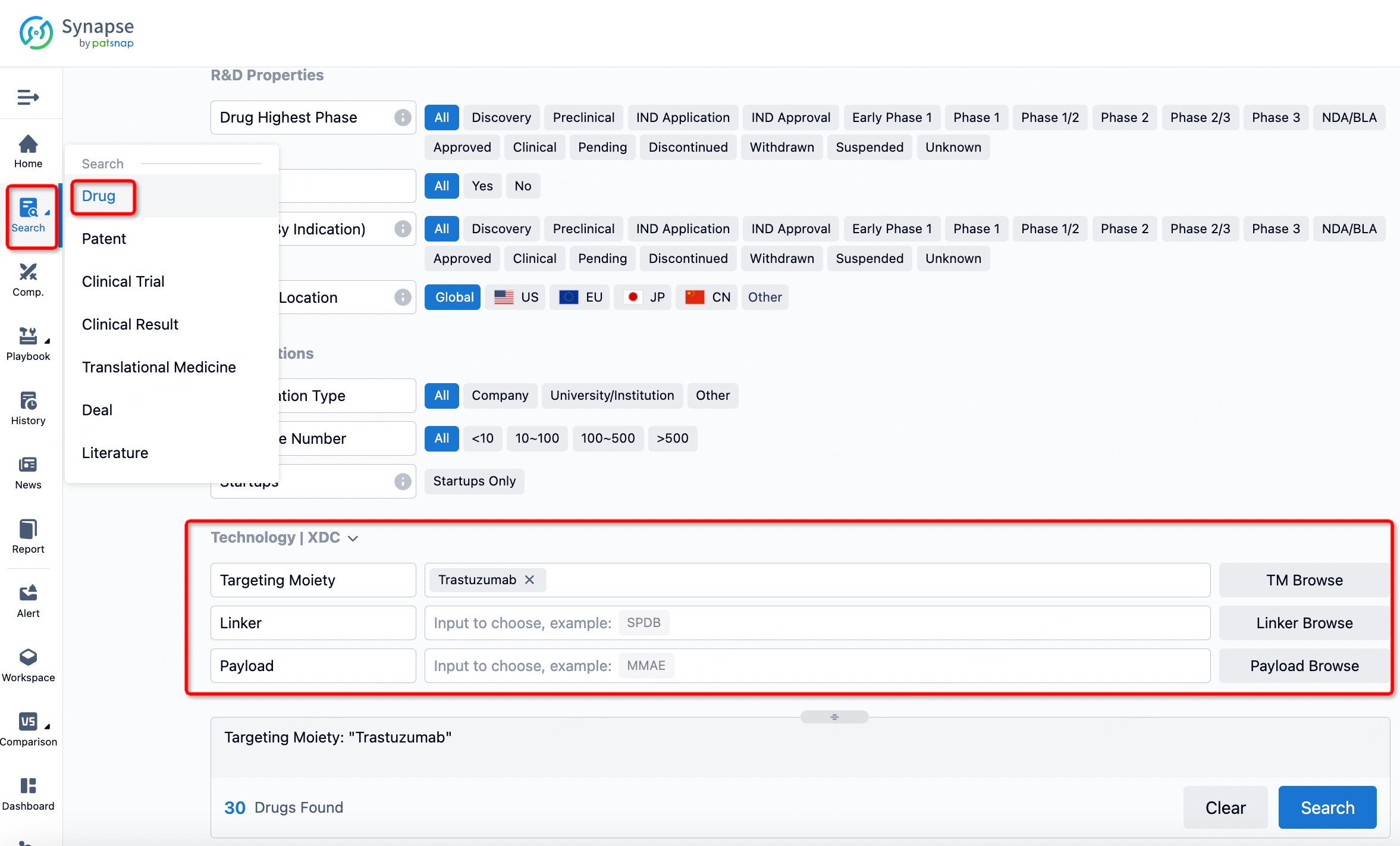
If you want to learn about the latest developments in ADC drugs, you can use the drug search module of the Synapse database. This module supports searching for ADC drugs by classification through Targeting Moiety, Linker, and Payload.
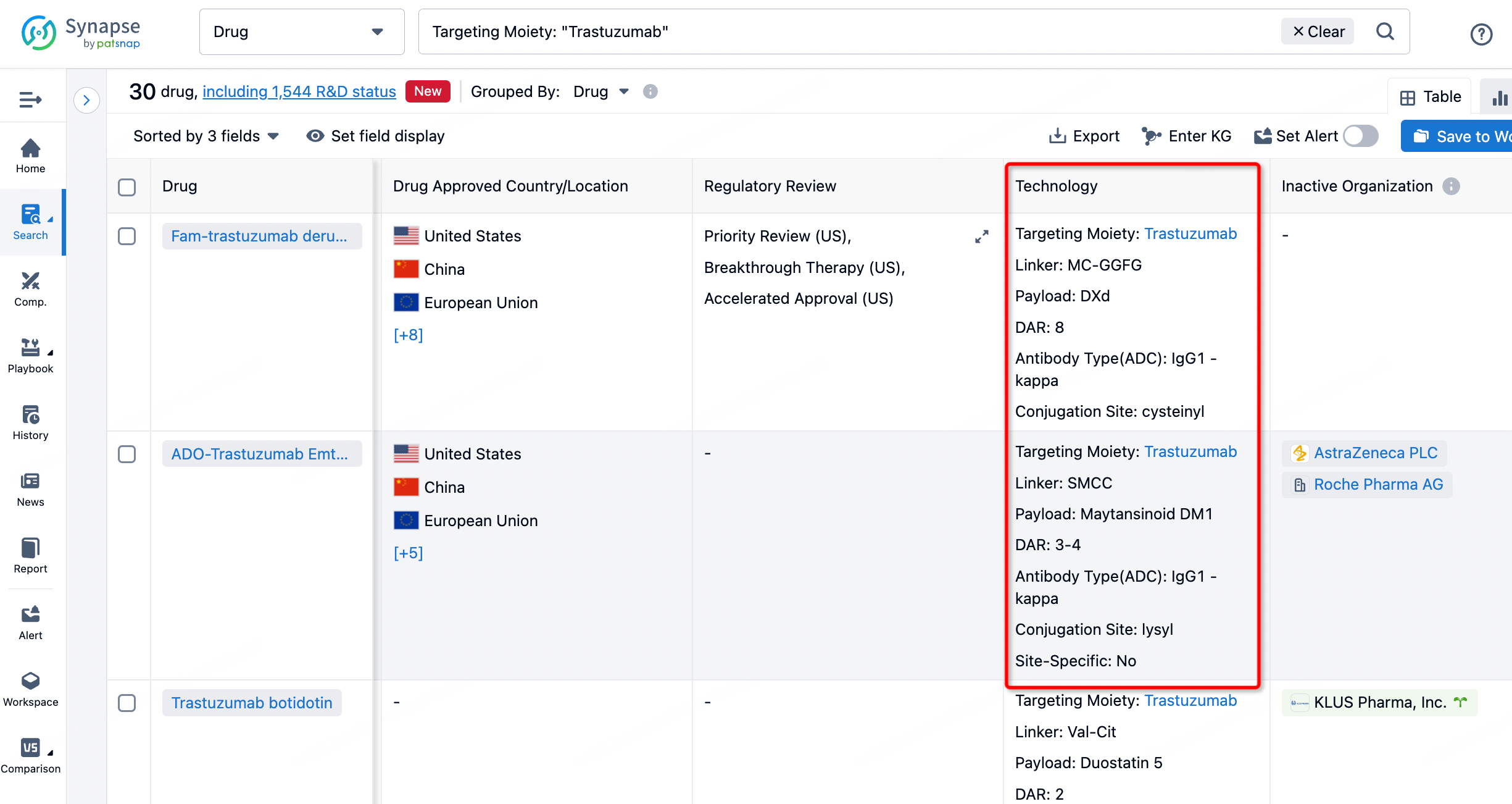
On the search results page, you can easily review information related to the ADC's technical category of the drugs.
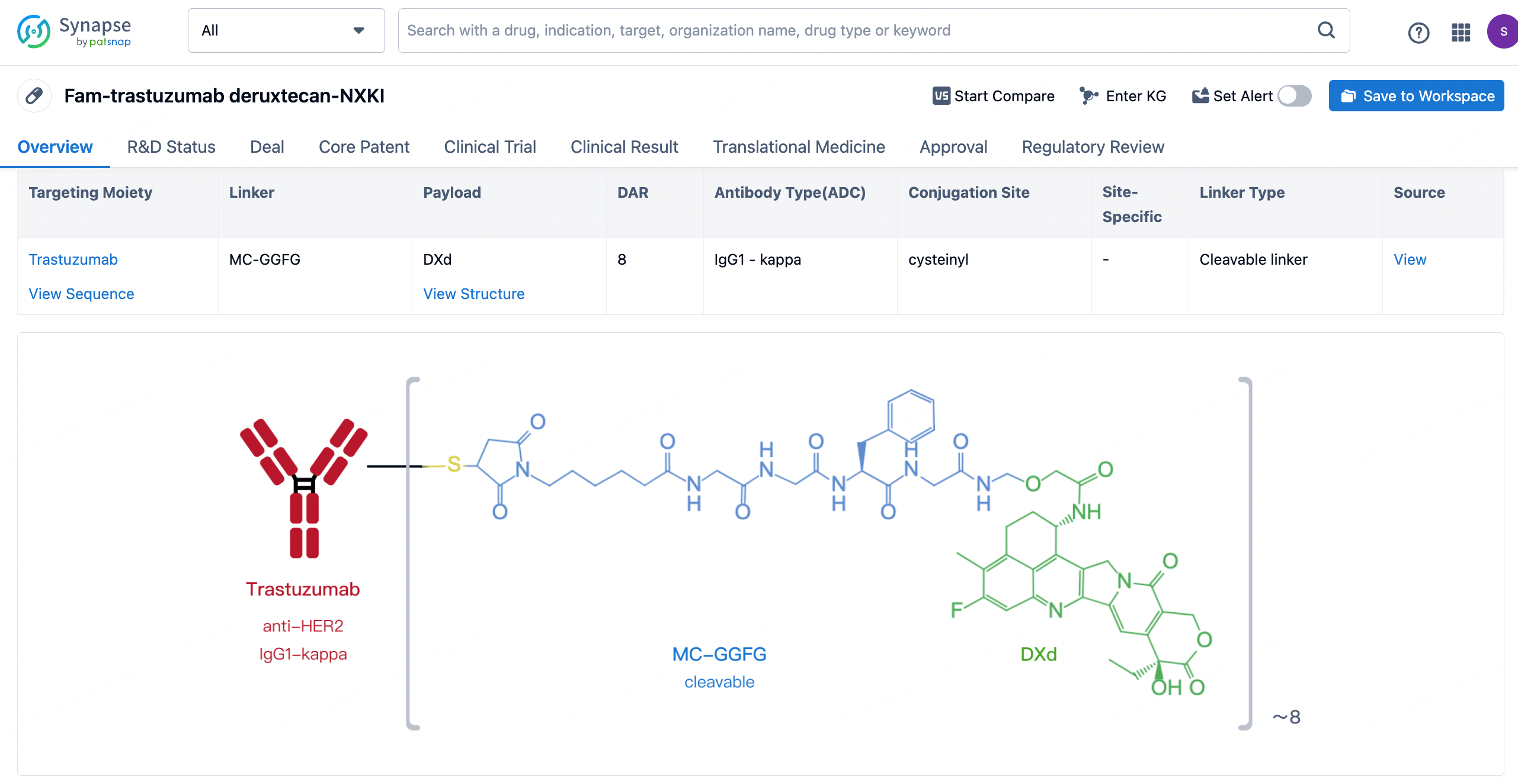
After clicking to enter the drug details page, you can also effortlessly obtain structural information about the ADC drug.
Click on the image below to embark on a brand new journey of drug discovery!