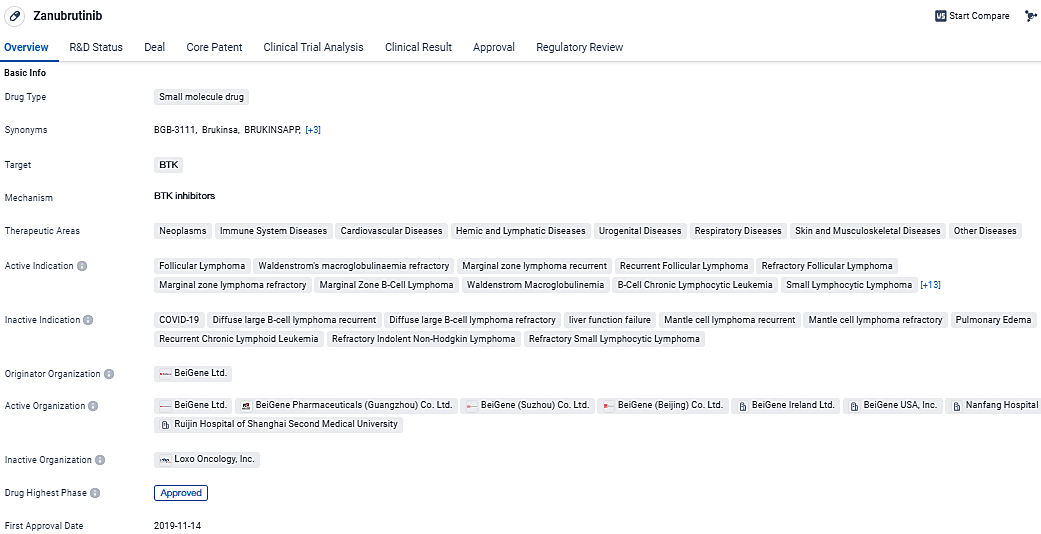
BeiGene Reveals Swift FDA Nod for BRUKINSA Use in Recurrent or Stubborn Follicular Lymphoma Cases
BeiGene, Ltd., a worldwide biopharmaceutical firm focused on cancer, has disclosed that the U.S. Food and Drug Administration provided expedited authorization for their product BRUKINSA(zanubrutinib). This authorization is for its use in treating adult patients who have undergone a relapse or are resistant to follicular lymphoma. It is intended for use in conjunction with obinutuzumab, an anti-CD20 monoclonal antibody, following failure of at least two previous systemic therapies. The conditional approval is premised on the observed response rates and the sustained effectiveness of the response. This approval signifies the fifth indication for BRUKINSA within the realm of B-cell malignancies in the United States.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The recent authorization of BRUKINSA marks a significant breakthrough by providing the first and unique BTK inhibitor therapeutic option for individuals in the U.S. suffering from follicular lymphoma who have not seen success with initial treatments or have endured a recurrence. "BRUKINSA stands alone as the sole BTK inhibitor proven effective against this particular cancer, now with the most comprehensive indication coverage, encompassing five distinct cancer uses, seen within its drug category across the globe," stated Mehrdad Mobasher, M.D., M.P.H., Chief Medical Officer, Hematology at BeiGene. "The unique clinical properties of BRUKINSA and our dedication to offer this crucial therapy to patients internationally is evident by this expansion," Mehrdad Mobasher remarked further.
The FDA's expedited approval program sanctioned BRUKINSA for R/R FL treatment, predicated on the observed overall response rate from the pivotal ROSEWOOD trial, as evaluated by an autonomous review committee. The continuing endorsement for this use hinges on forthcoming confirmation and detailed clinical benefit demonstration in the MAHOGANY trial currently in progress. Additionally, the FDA has conferred upon the R/R FL application both Fast Track Designation and Orphan Drug Designation.
With authorization in 70 markets across the globe which include the U.S., EU, Great Britain, Canada, Australia, China, South Korea and Switzerland for certain conditions, BRUKINSA is expanding its reach. At the same time, its development for more potential uses is ongoing internationally. The worldwide BRUKINSA development effort has encompassed over 5,000 individuals across 29 countries and regions.
As a targeted therapy, BRUKINSA is a small molecule that blocks Bruton’s tyrosine kinase, engineered for consistent and thorough BTK protein inhibition by honing bioavailability, lifespan, and specificity. Demonstrating a pharmacokinetic profile distinct from other approved BTK inhibitors, BRUKINSA has shown effectiveness in halting the growth of malignant B cells in several tissues relevant to the disease.
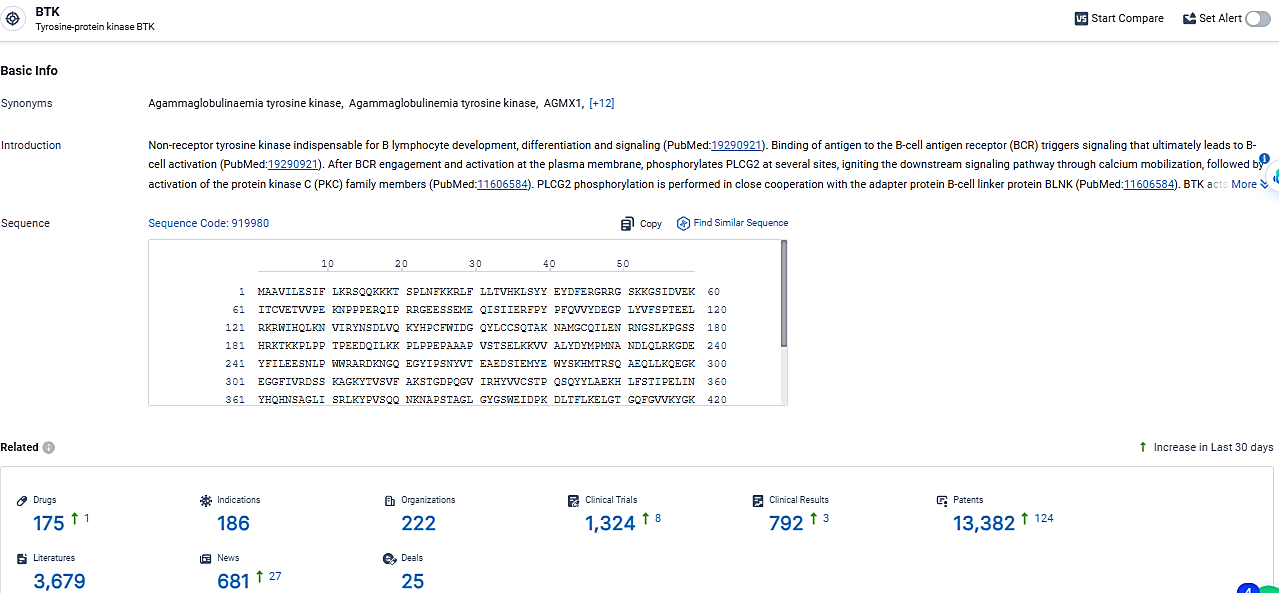
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 11, 2024, there are 175 investigational drugs for the BTK target, including 186 indications, 222 R&D institutions involved, with related clinical trials reaching 1324, and as many as 13382 patents.
Zanubrutinib is a small molecule drug that targets BTK and has shown promising results in the treatment of various diseases, particularly in the field of oncology. Its approval in multiple therapeutic areas and its regulatory designations reflect its potential as a valuable treatment option for patients. The drug's development by BeiGene Ltd. further underscores the company's commitment to advancing innovative therapies in the pharmaceutical industry.