BeiGene's Patent Research and Practical Operation Guide for Tislelizumab (3)
Based on the characteristics of monoclonal antibody drugs, we have summarized the process for conducting patent research.You can start by reading the following article to get background information.
BeiGene's Patent Research and Practical Operation Guide for Tislelizumab (1)
BeiGene's Patent Research and Practical Operation Guide for Tislelizumab (2)
Retrieve CDR Sequences to Find All Patents Related to Tislelizumab
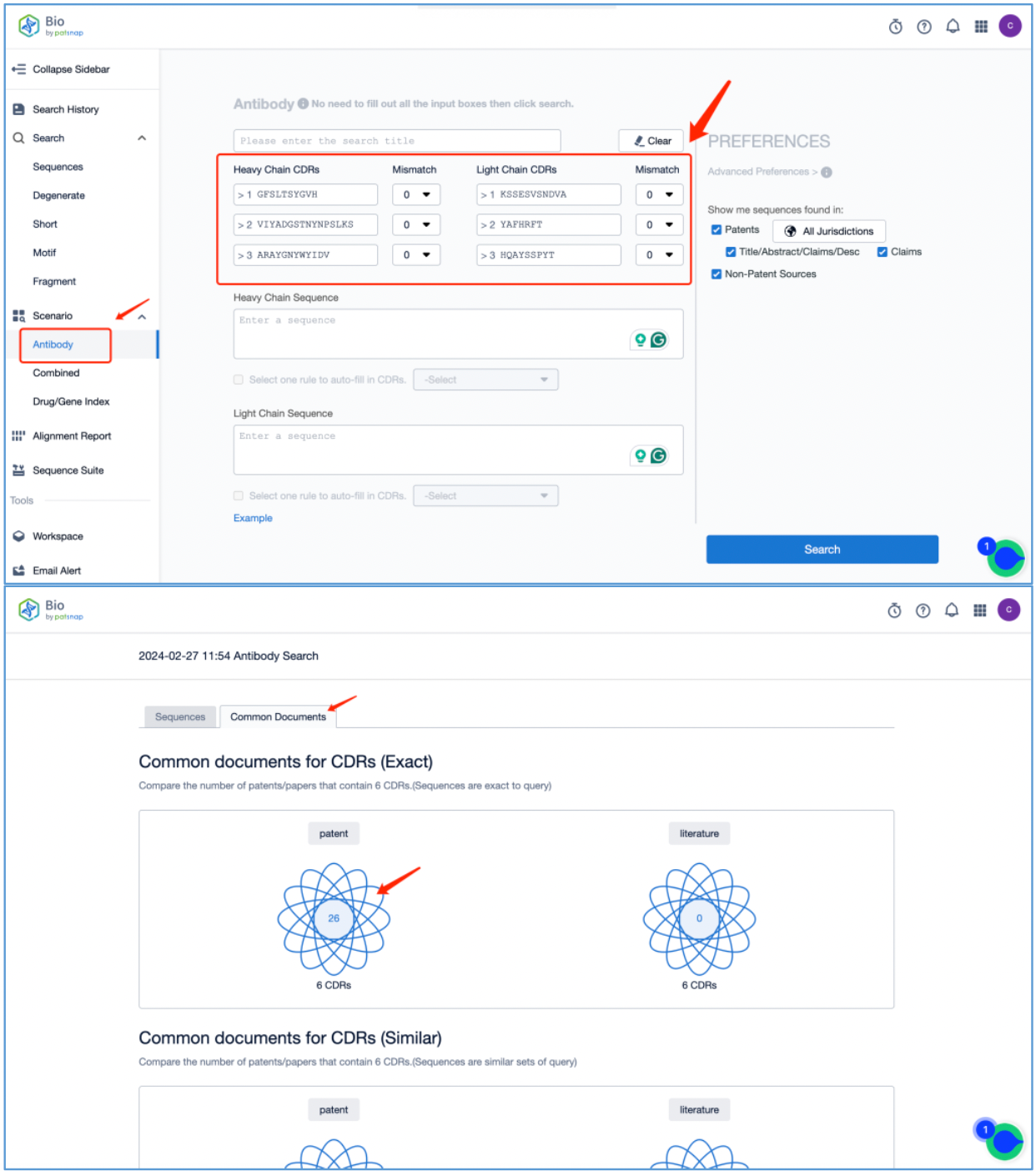
First, log in to the Patsnap Bio database. Select the antibody search option from the search menu on the left side of the Bio database homepage. Then, fill in the heavy chain CDRs and light chain CDRs. Perform an exact search by entering 0 for the mismatch number. Click on "Search." The resulting antibody report provides 26 patents that contain 6 exact public heavy and light chain CDRs, located under Common Documents.
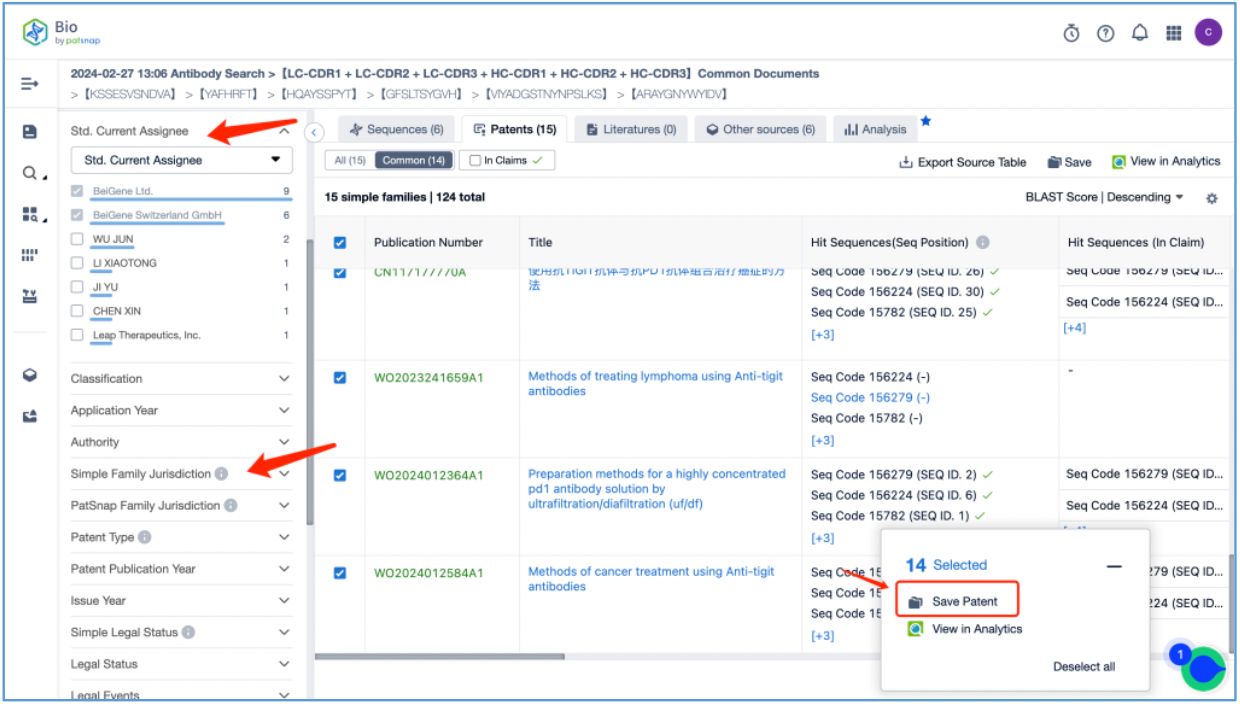
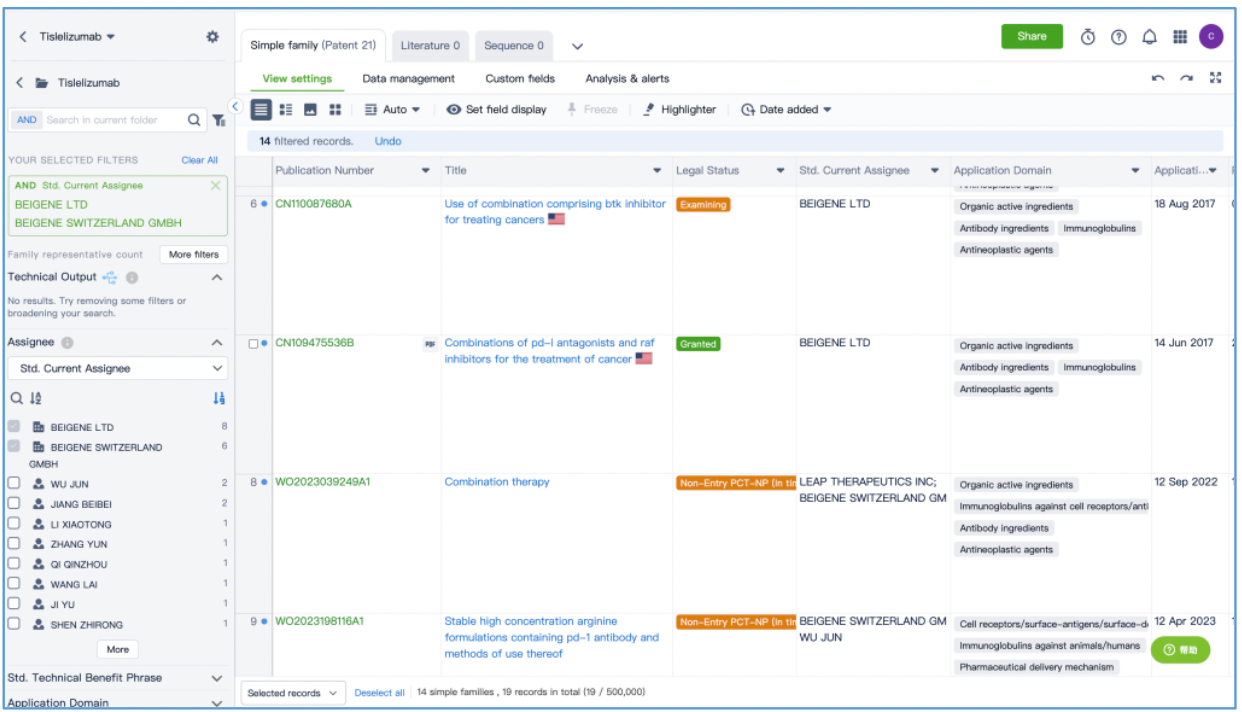
Next, filter the 26 patent results to only include those where the current patent holder is "BeiGene and its subsidiaries." Save the resulting 14 simple family patents in the "tislelizumab" Workspace. This process reveals an additional 7 simple family patents. Therefore, after conducting a search in Patsnap Bio, we discovered an additional 7 simple family patents beyond the 7 found in the Synapse database search. This demonstrates the benefit of using both Bio and Synapse databases to comprehensively search for antibody patents.
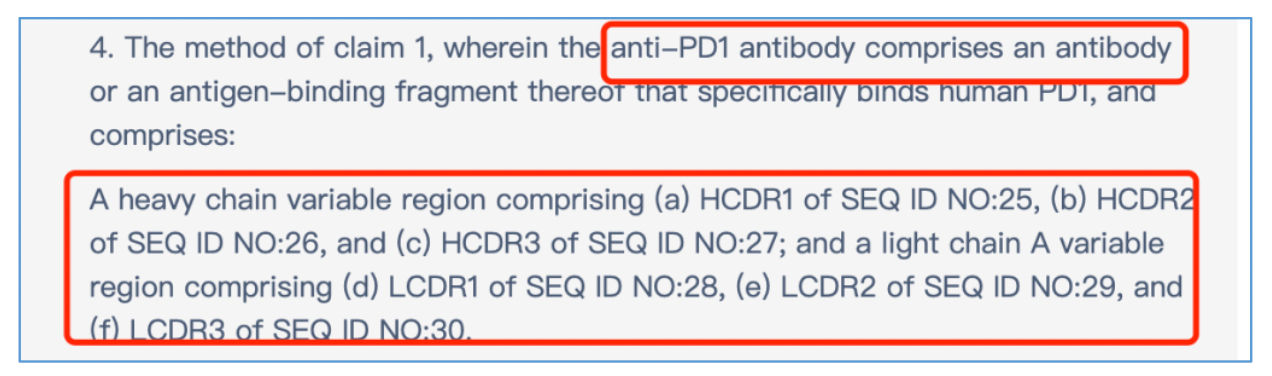
Finally, one of the patents explicitly specifies a drug combination patent with CDR sequences, which can be used to verify if the analyzed CDR sequences match those of the drug itself. For instance, among the 14 collected patents, patent number 2022800108057, titled "Methods of treating cancer using a combination of anti-TIGIT antibodies and anti-PD1 antibodies" specifies the sequence of the combined anti-PD1 antibody in claim 4. By comparing this with the CDRs, it was confirmed that our analyzed CDRs match those of tislelizumab, confirming their authenticity as the drug's CDR sequences.
Analyze all Patents Related to Tislelizumab
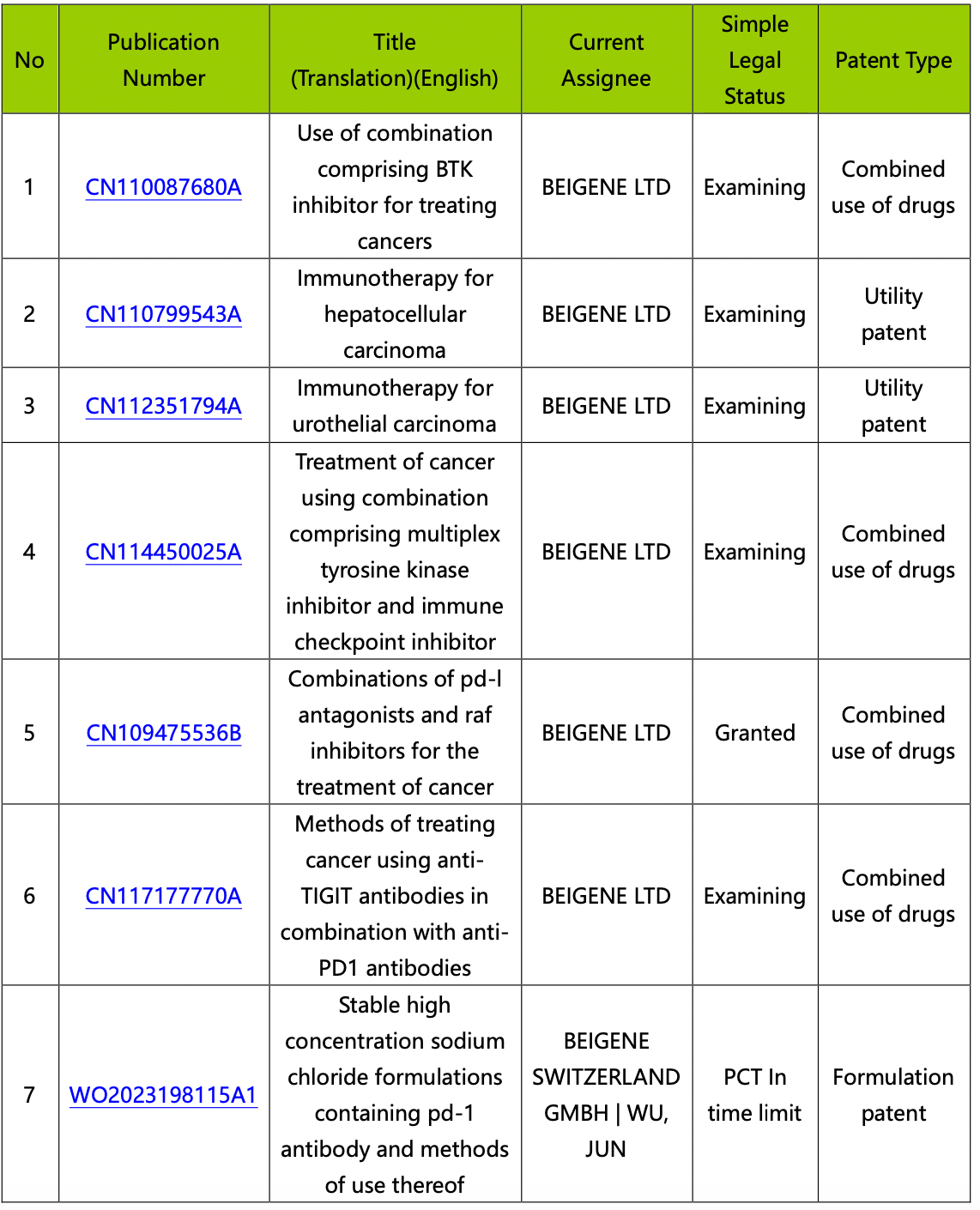
This stage is to analyze the 14 simple family patents saved in the "tislelizumab" workspace. You can choose to view and analyze the patents online in the workspace, or export all patents for offline detailed analysis. After conducting a patent viewing analysis, categorize the patents according to their protection types, as demonstrated in the table below.
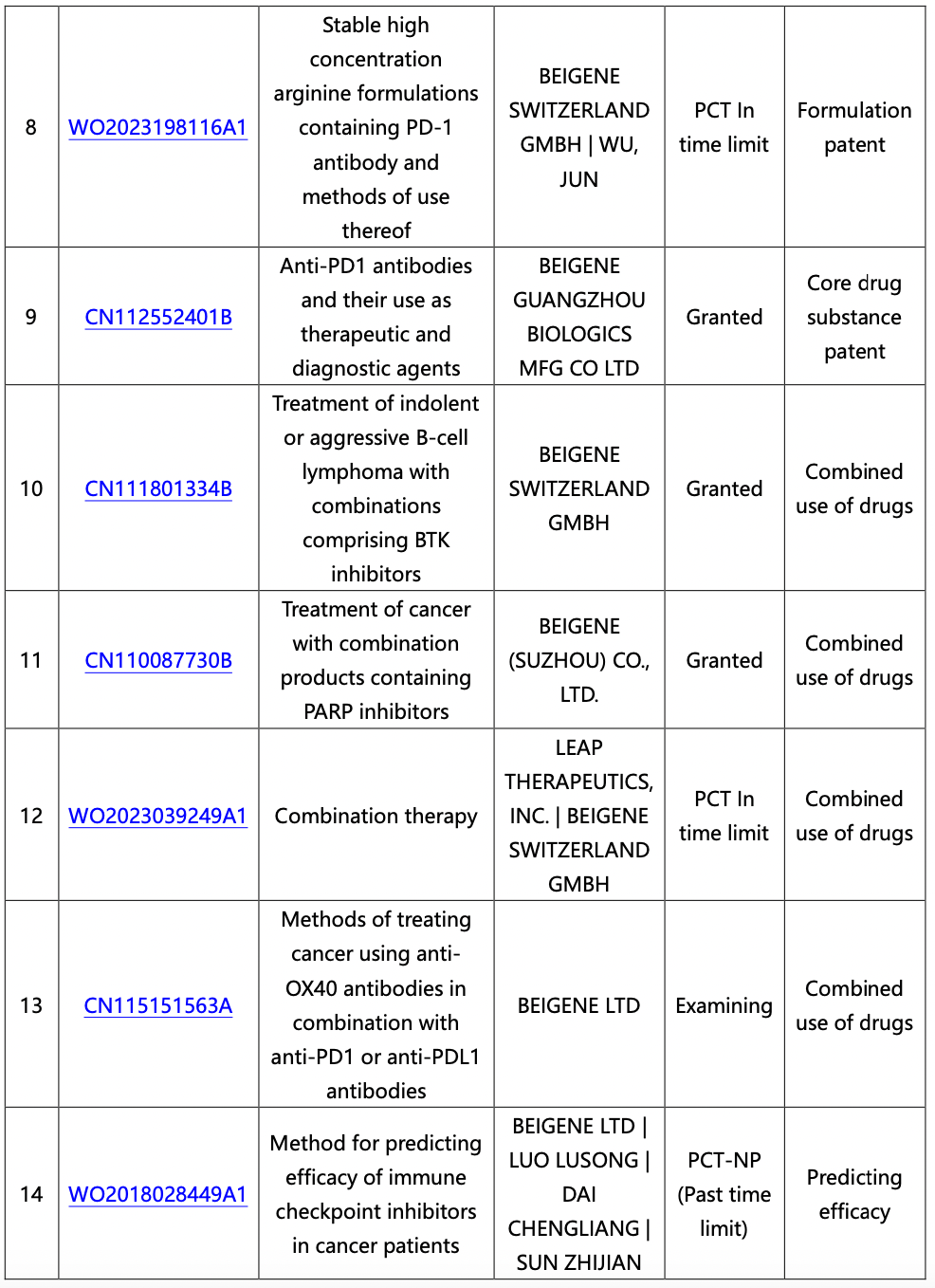
The core substance patent CN112552401B (anti-PD1 antibody and its use as a therapeutic and diagnostic agent) was filed on September 13, 2013, published on March 19, 2015, and granted on August 25, 2023, without any priority date. There are a total of 6 patents in the same family authorized domestically, namely CN112552401B, CN107011441B, CN107090041B, CN108715615B, CN112457403B, and CN105531288B. The protection points include nucleotides encoding PD-1 antibody or antigen fragments, nucleotides encoding PD-1 monoclonal antibody, PD-1 monoclonal antibody, vector, host cell, and preparation method for the protection points of CN112552401B. The protection points for CN107011441B include monoclonal antibody and utility. The protection points for CN107090041B include antibody or its fragments. The protection points for CN108715615B include monoclonal antibody or its fragments. The protection points for CN112457403B include utility. The protection points for CN105531288B include PD-1 antibody or its antigen-binding fragment, constant domain, and utility.
Utility patent CN110799543A (Immunotherapy for hepatocellular carcinoma), has the earliest priority date of June 26, 2017, application date of June 26, 2018, and publication date of January 3, 2019. This patent has not been granted domestically, with a total of 1 patent in the same family domestically published. The specific protection point is for the treatment of hepatocellular carcinoma (HCC).
Utility patent CN112351794A (Immunotherapy for urothelial carcinoma), has the earliest priority date of February 9, 2018, application date of February 8, 2019, and publication date of August 15, 2019. This patent has not been granted domestically, with a total of 1 patent in the same family domestically published. The specific protection point is for the treatment of urothelial carcinoma.
Formulation patent WO2023198115A1 (Stable high concentration sodium chloride formulations containing pd-1 antibody and methods of use thereof), has the earliest priority date of April 14, 2022, application date of April 12, 2023, and publication date of October 19, 2023. This patent has not been granted domestically, with a total of 1 patent in the same family publicly available. The specific protection points are for stable high-concentration sodium chloride formulation and preparation method.
Formulation patent WO2023198116A1 (Stable high concentration arginine formulations containing PD-1 antibody and methods of use thereof), has the earliest priority date of April 14, 2022, application date of April 12, 2023, and publication date of October 19, 2023. This patent has not been granted domestically, with a total of 1 patent in the same family publicly available. The specific protection point is for stable high-concentration arginine formulation.
Patent Layout Diagram for the Clinical Timeline of Producing Tislelizumab Monoclonal Antibody
Create a timeline of BeiGene's 14 patents related to tislelizumab monoclonal antibody according to the clinical timeline outlined and annotate the patent status based on the protection points in the claims of substance patents, utility patents, and formulation patents, following the patent status in mainland China. After labeling the patent types, the resulting patent layout diagram is as follows:
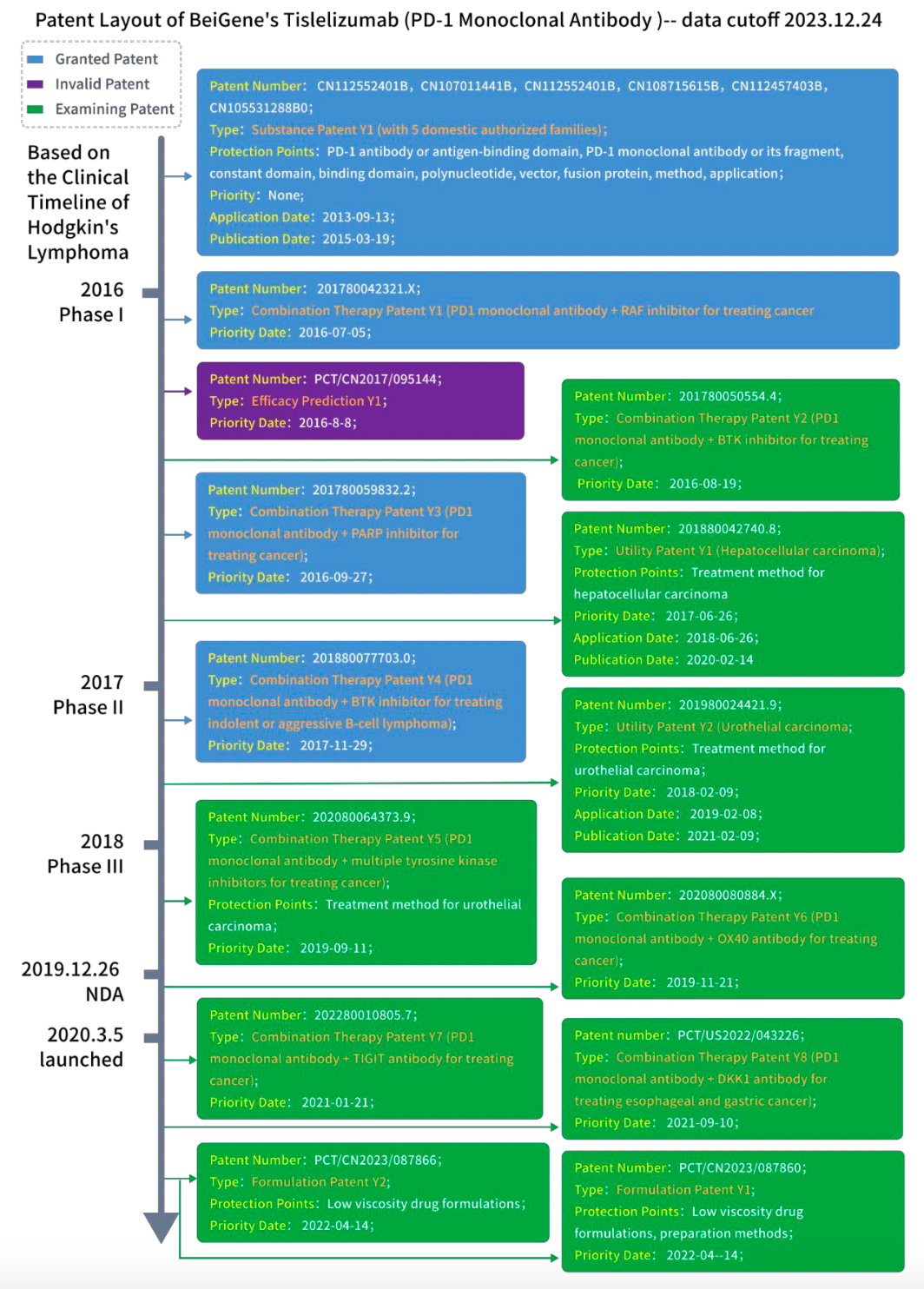
By examining the patent layout diagram:
1. It becomes apparent that BeiGene currently holds authorization for 4 basic family patents in mainland China. Additionally, one of these patents has expired, while 9 others are currently under review. This data offers insights into BeiGene's current protection strategies for tislelizumab, enabling a focused analysis of the extent of protection.
2. A fundamental substance patent was secured prior to the commencement of clinical trials, which has since been subdivided into multiple cases. Presently, there are 6 domestically authorized family patents stemming from the core substance patent. This exemplifies the ongoing expansion of the core substance patent's coverage, bolstering its protective scope.
3. Moreover, there are 8 simple family patents related to the combined use of tislelizumab, underscoring the enhancement of product viability through combination therapies. Following the product launch, an additional 2 simple family patents pertain to formulation types, with the aim of prolonging patent protection by introducing new formulations in later stages.
4. Most of the patent layout leverages priority rights to extend the duration of patent protection. The time gap between priority patents and formal patents is nearly at a 1-year threshold, thereby extending the overall period of patent protection.
5. It is evident that key types of patents crucial throughout the drug's lifecycle include substance patents, utility patents, combination therapy patents, and new formulation patents.
6. Furthermore, a more in-depth analysis can be conducted on the efficacy support data within each patent to evaluate the scope of patent examination.
Summary
This report outlines the process of monoclonal antibody drug patent research with the investigation of BeiGene patent portfolio related to its tislelizumab monoclonal antibody. Throughout the retrieval process, various online tools such as Patsnap Analytics, Patsnap Synapse, Patsnap Bio, and other resources were utilized. This showcases that patent research is a multi-stage, collaborative endeavor, typically encompassing patent retrieval, patent screening, drug clinical analysis, core patent examination, and other essential stages.
The tislelizumab patent layout diagram offers comprehensive insight into BeiGene's patent protection strategy while reflecting on industry R&D trends. This serves as a valuable intelligence tool for enterprise infringement analysis, biosimilar drug development, investment and financing decisions, and drug R&D strategies to enhance pharmaceutical IP and advance new therapies to market.
For more information, please click the image link below to access the full report.




