UCB Releases Four-Year BIMZELX® Data for Severe Plaque Psoriasis at EADV 2024
UCB, an international biopharmaceutical company, disclosed new four-year data from patients with moderate-to-severe plaque psoriasis treated with BIMZELX® (bimekizumab-bkzx)), a dual inhibitor targeting interleukin (IL)-17A and IL-17F. These post-hoc analyses reveal the sustained response over four years in patients who achieved nearly complete or complete skin clearance after one year of treatment with BIMZELX. The data also include clinical responses over four years in patients who were switched to BIMZELX after insufficient response to adalimumab, ustekinumab, or secukinumab.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Presented at the 33rd European Academy of Dermatology and Venereology (EADV) Congress in Amsterdam, Netherlands, from September 25–28, 2024, these findings highlight significant research progress. A focal point is the BE UNIQUE study, a multicenter, open-label Phase 3b research initiative aimed at evaluating the rapid onset, high efficacy, and longevity of clinical and molecular responses in psoriatic disease patients.
"Considering the persistent nature of psoriasis, assessing long-term treatment effects is crucial. Attaining fully clear skin remains a primary objective for individuals with moderate-to-severe plaque psoriasis. Data introduced at EADV 2024 indicated that over 70% of patients who achieved total skin clearance after one year preserved this outcome at the four-year mark," stated Professor Richard Warren from Northern Care Alliance NHS Foundation Trust and The University of Manchester, UK. "The four-year results disclosed at EADV 2024 authenticate the sustained complete skin clearance for patients on bimekizumab treatment," added Fiona du Monceau, Executive Vice President, Head of Patient Evidence, UCB. "We are also excited to unveil the design framework of BE UNIQUE, a Phase 3b study poised to determine if the durability of clinical response to bimekizumab correlates with molecular and cellular changes in the skin, blood, and joints of psoriatic disease patients."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
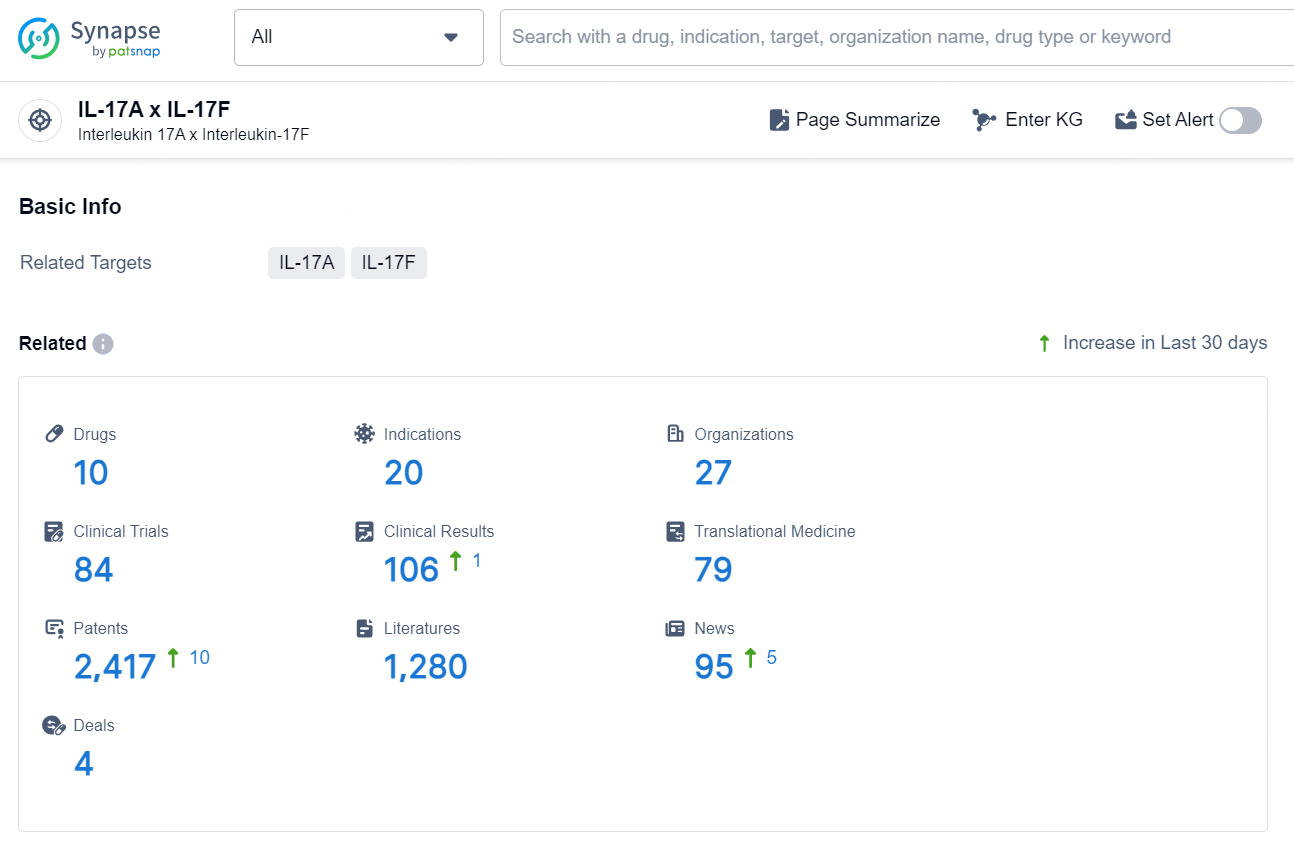
According to the data provided by the Synapse Database, As of September 24, 2024, there are 10 investigational drugs for the IL-17A and IL-17F targets, including 20 indications, 27 R&D institutions involved, with related clinical trials reaching 84, and as many as 2413 patents.
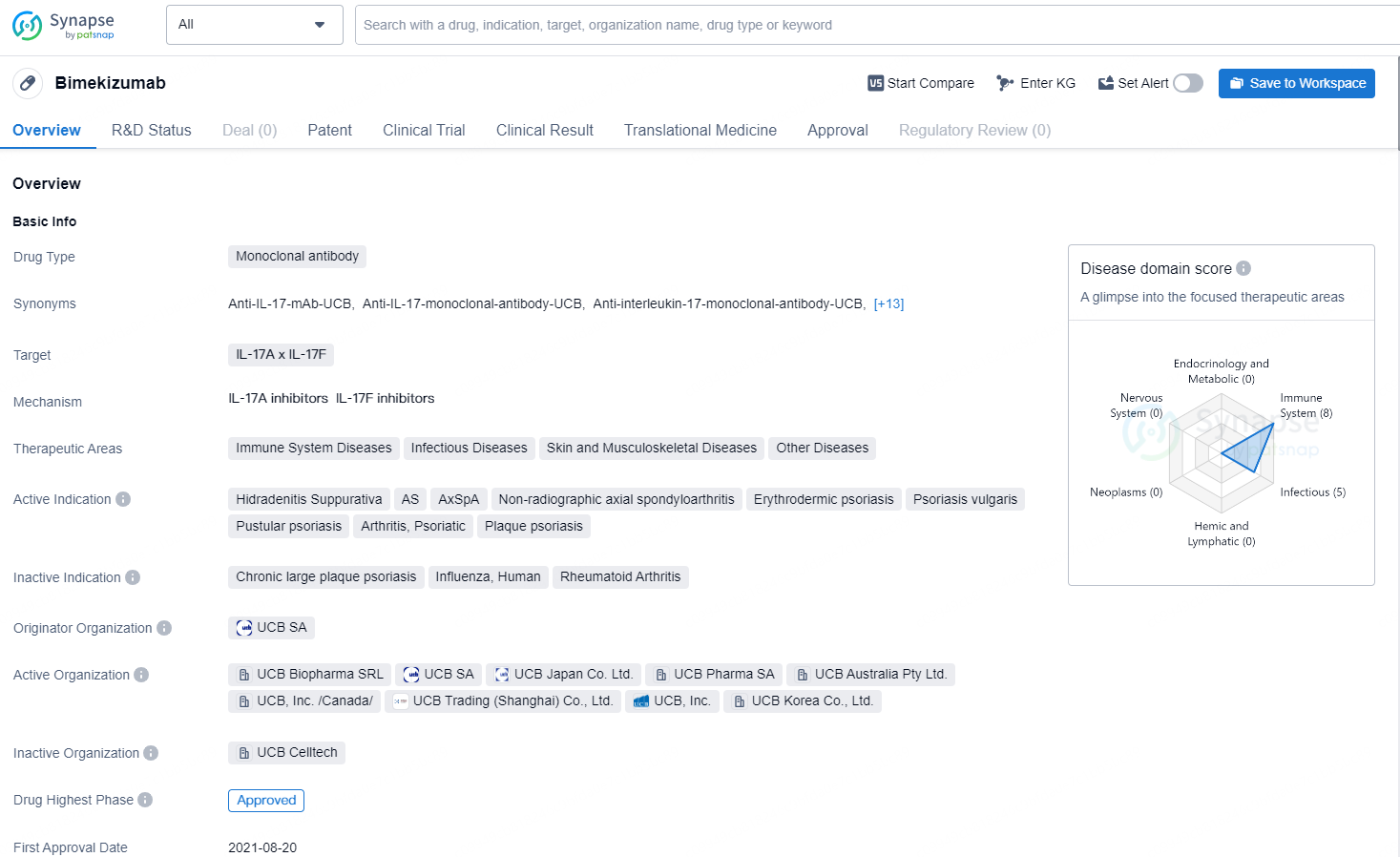
Bimekizumab is a monoclonal antibody drug that targets IL-17A and IL-17F. It is indicated for the treatment of various immune system diseases, infectious diseases, skin and musculoskeletal diseases, and other conditions. The active indications for Bimekizumab include Hidradenitis Suppurativa, Ankylosing Spondylitis (AS), Axial Spondyloarthritis (AxSpA), Non-radiographic axial spondyloarthritis, Erythrodermic psoriasis, Psoriasis vulgaris, Pustular psoriasis, Psoriatic Arthritis, and Plaque psoriasis.