BeiGene's Patent Research and Practical Operation Guide for Tislelizumab (2)
Based on the characteristics of monoclonal antibody drugs, we have summarized the process for conducting patent research.You can start by reading the following article to get background information.
BeiGene's Patent Research and Practical Operation Guide for Tislelizumab (1)
Tislelizumab, referred to as bazeleus, represents a humanized IgG4 monoclonal antibody targeting PD-1. On December 26, 2019, it received market launch approval from the Chinese National Medical Products Administration (NMPA).
Tislelizumab represents an immune checkpoint inhibitor, categorized as a biologic agent, specifically engineered to treat diverse forms of cancer. By impeding the interaction between PD-1 and PD-L1, tislelizumab monoclonal antibody triggers the immune response of T cells, facilitating the eradication of cancer cells. This mechanism underscores its pivotal role in harnessing the body's immune system to combat cancerous growths effectively. Tislelizumab's targeted approach not only addresses the underlying mechanisms of cancer immune evasion but also offers promise as a potent therapeutic intervention in oncology.
In clinical settings, the utilization of tislelizumab monoclonal antibody has become increasingly prevalent for the treatment of several malignancies, notably non-small cell lung cancer (NSCLC) and hepatocellular carcinoma (HCC). This biologic agent has demonstrated remarkable efficacy in targeting cancerous cells, particularly in the advanced stages of the disease. For patients with advanced cancer, especially following rounds of chemotherapy and radiotherapy, the integration of tislelizumab monoclonal antibody into treatment regimens offers a promising avenue for enhancing survival rates.
Tislelizumab stands out as a flagship product within BeiGene's portfolio, having secured approval in advance of its counterparts in the European Union, and it is administered as a second-line therapy for adult patients diagnosed with esophageal squamous cell carcinoma (ESCC).
As of the third quarter of 2023, tislelizumab monoclonal antibody has secured approval from the China National Medical Products Administration (NMPA) for 11 indications in China, with 9 of these indications integrated into the national medical insurance drug list. During the third quarter, the sales revenue of the drug in China reached 1.046 billion yuan, marking a notable increase of nearly 19% compared to 879 million yuan recorded during the corresponding period the previous year. Combined with the sales figures of 1.836 billion yuan attained in the first half of the year, the cumulative domestic sales surpassed 2.8 billion yuan. This significant growth underscores the increasing recognition and demand for tislelizumab monoclonal antibody in the Chinese pharmaceutical market.
This guidebook will center on the examination and analysis of tislelizumab monoclonal antibody. The retrieved findings in this research effort extend up to December 24, 2023.
Retrieval of Basic Information on Tislelizumab
First, log in to Patsnap Synapse and search for "tislelizumab" from the homepage. Next, click on the retrieved tislelizumab drug card and navigate to the ‘Overview’ tab.
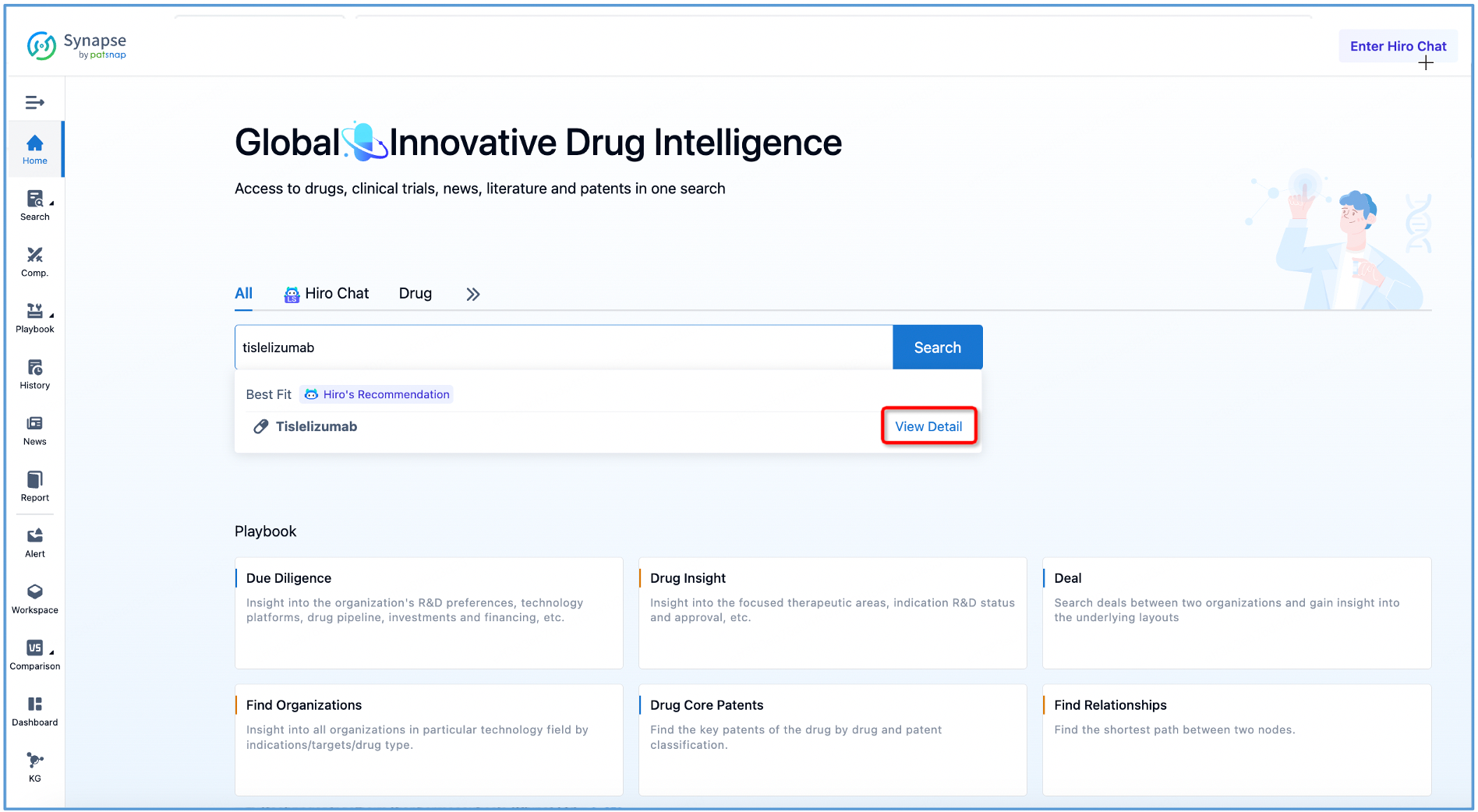
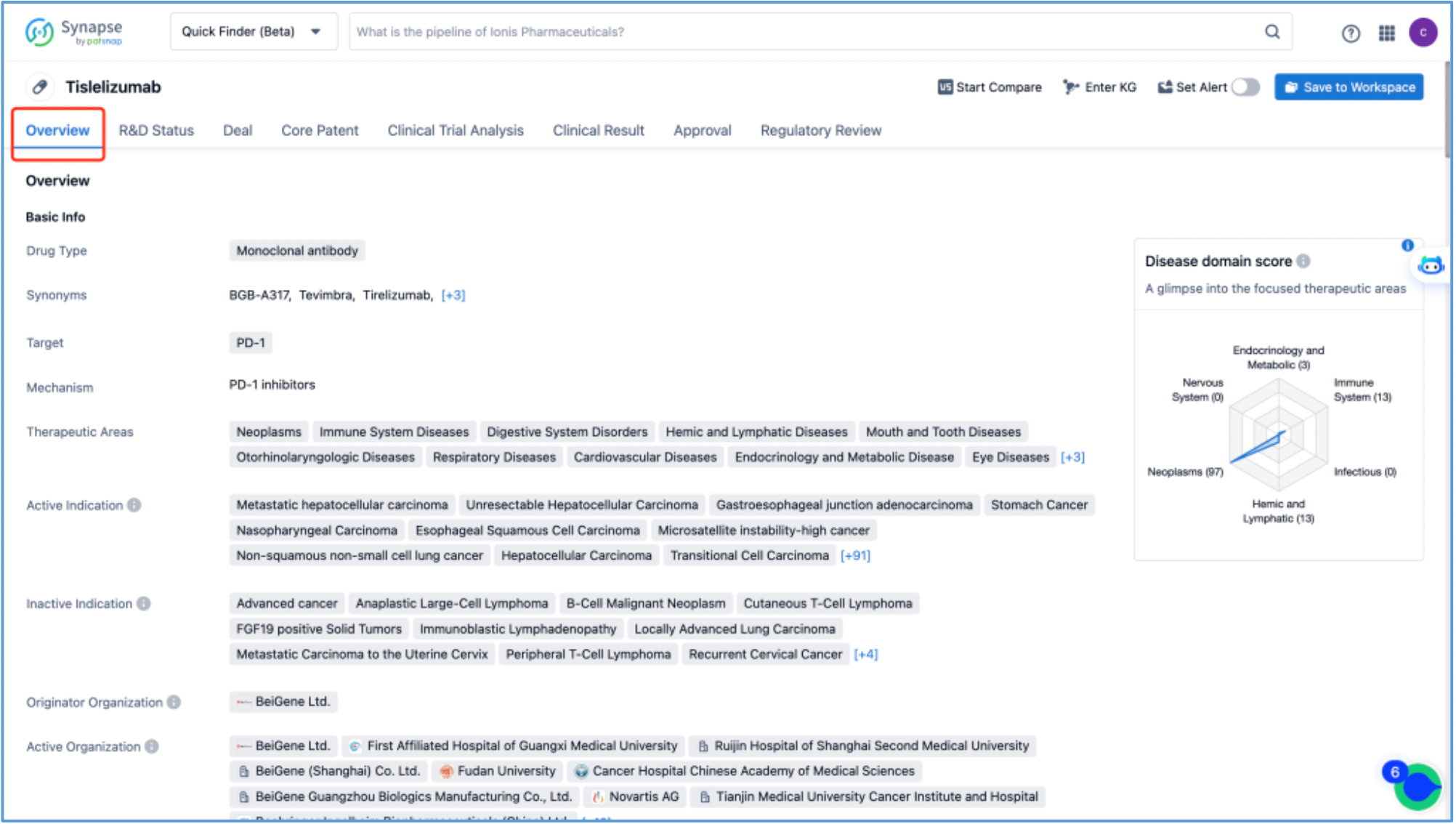
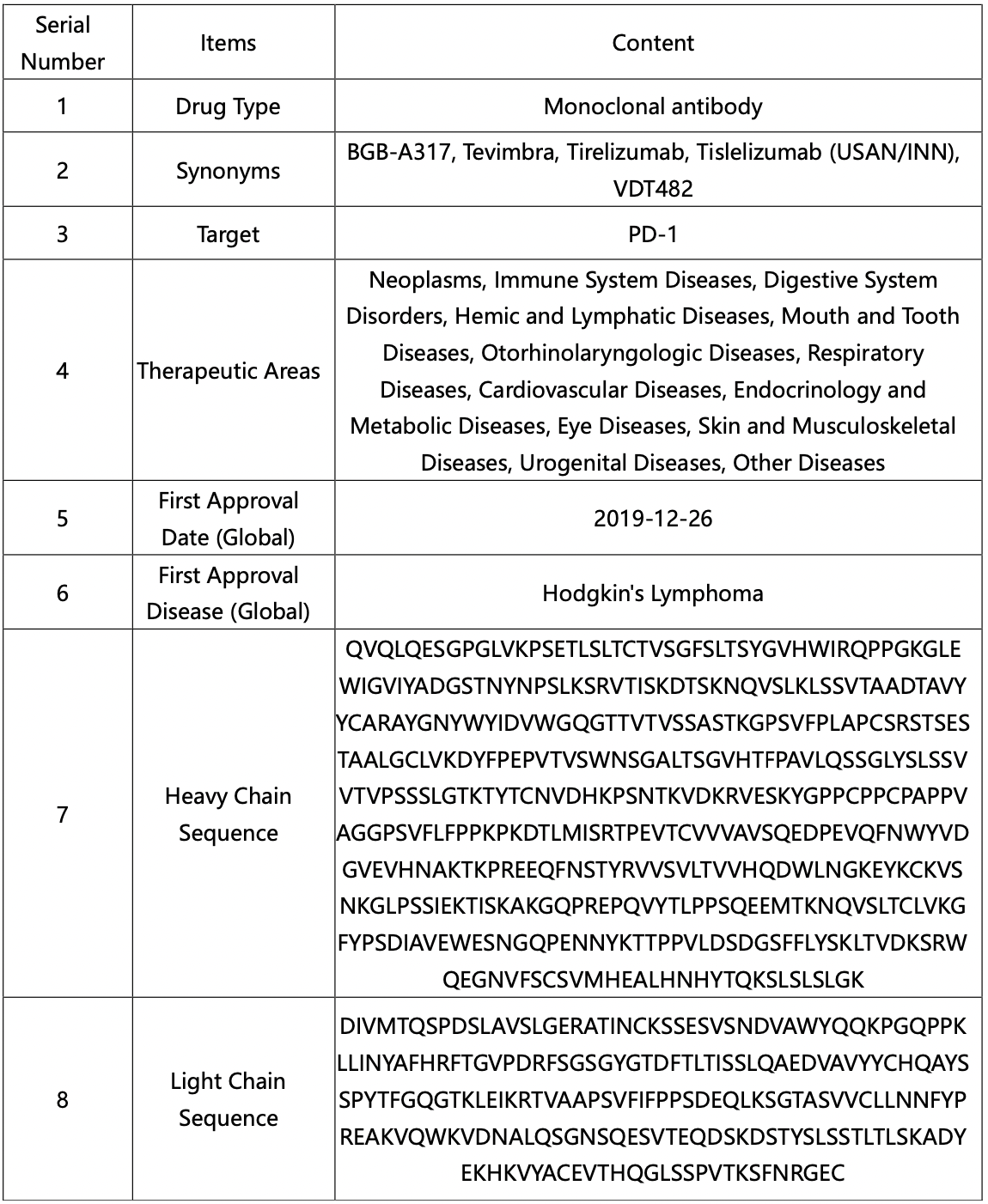
The compilation of fundamental data regarding tislelizumab
Gathering Clinical Data for Approved Indication “Hodgkin's Lymphoma” for Tislelizumab
Based on the foundational details outlined, it is evident that the first approval indication for tislelizumab is "Hodgkin's lymphoma." Consequently, the decision is to compile clinical data relevant to the treatment of "Hodgkin's lymphoma."
First, click on the R&D status, locate "Hodgkin's lymphoma," and then click on "View all clinical trials." Subsequently, you will only see data from Phase 2 and Phase 4 trials (since during NDA submission, which clinical results to declare for Phases 1, 2, and 3 as indications are considered internal data, the database displays public information accordingly). Therefore, after returning to the previous page, select "Clinical Trial Analysis" to confirm that the database currently only contains Phase 2 and Phase 4 clinical data for "Hodgkin's lymphoma," in addition to other clinical data such as solid tumors.
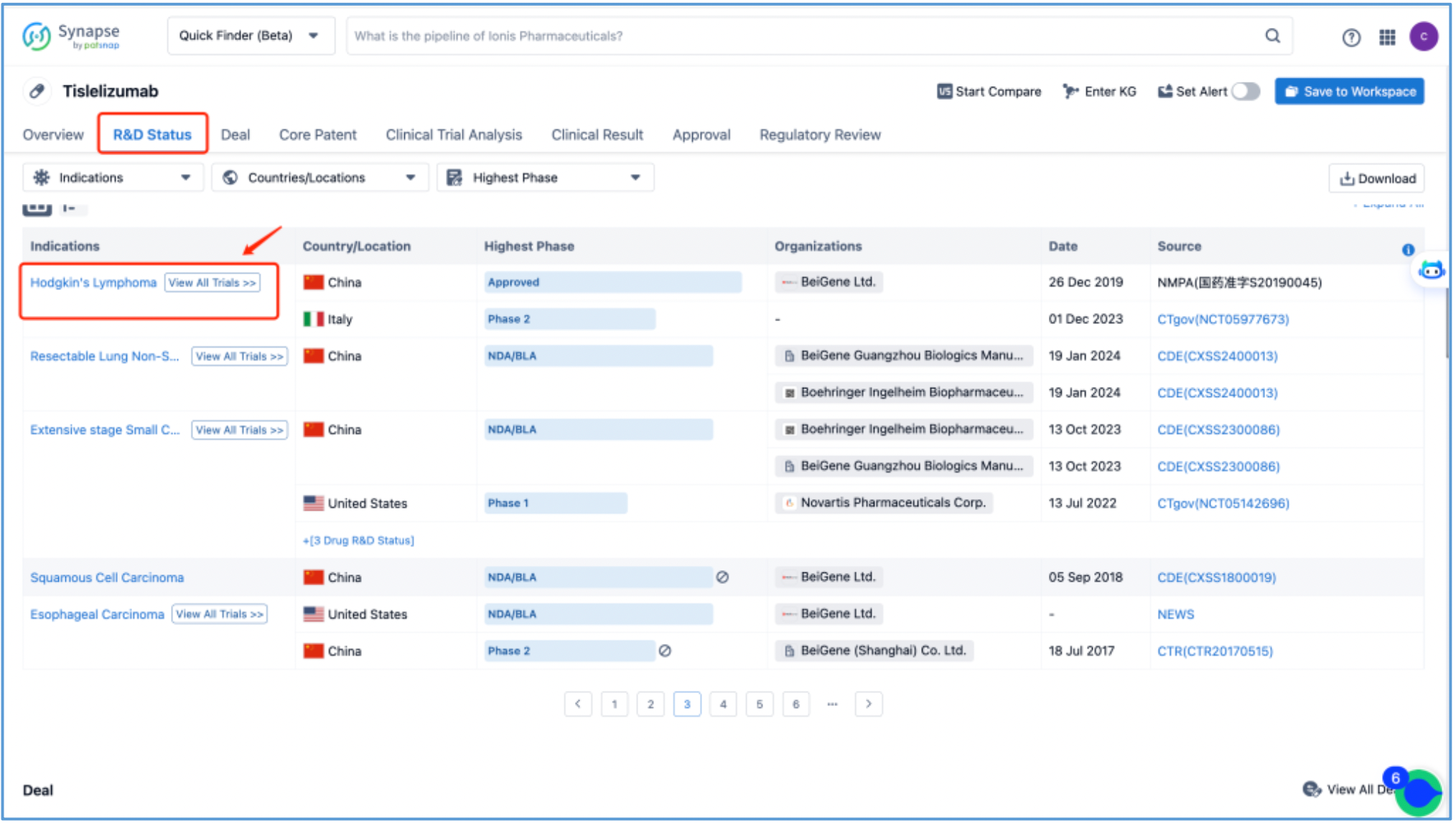
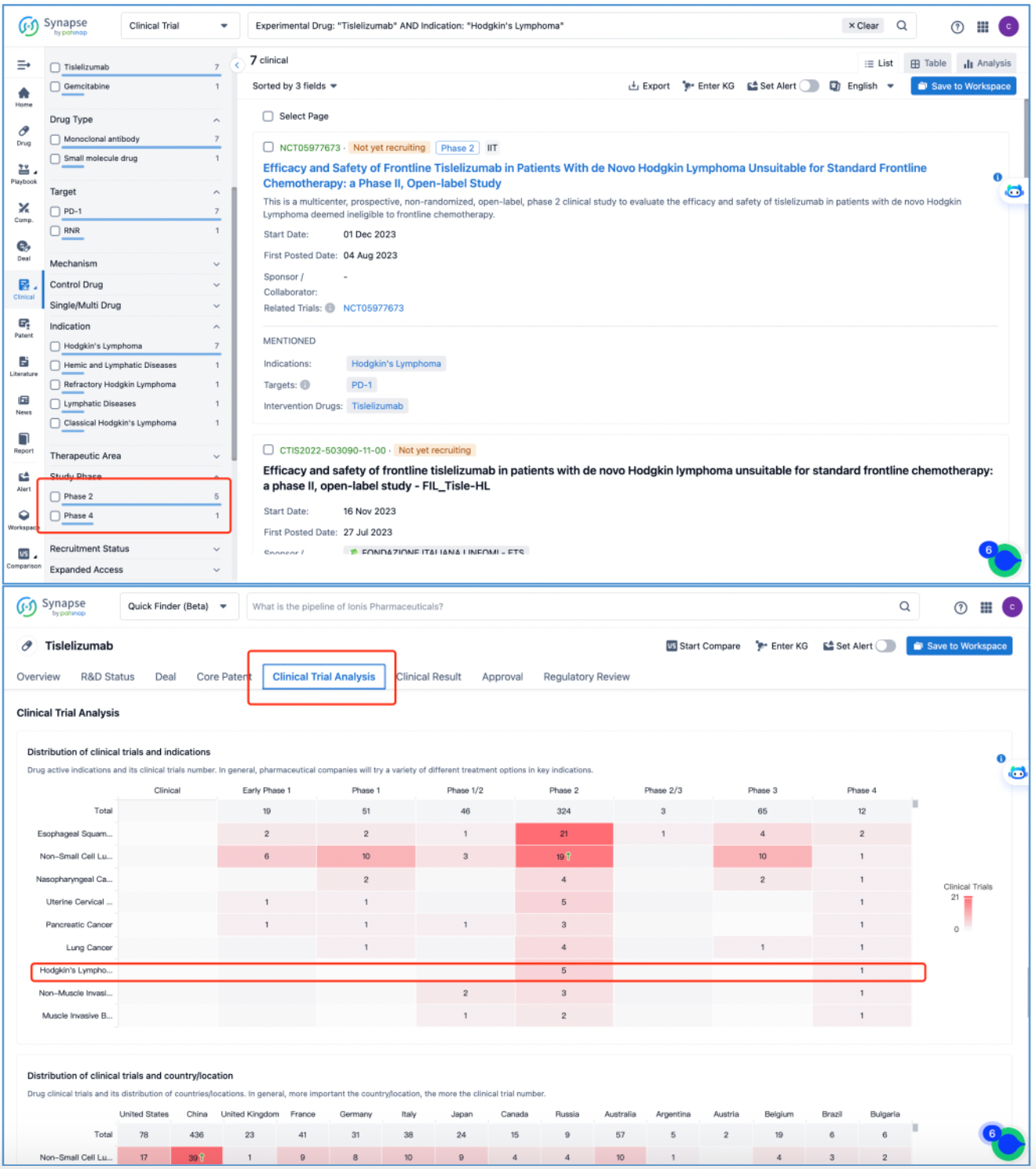
Therefore, to streamline the clinical timeline for the approval indication of tislelizumab for "Hodgkin's lymphoma," other than incorporating information from the database, we also integrated data from the CDE official website, disclosures from BeiGene, as well as various online news reports, ultimately finalizing the clinical timeline.
The finalized clinical information for the indication "Hodgkin's lymphoma" is presented in Table below:
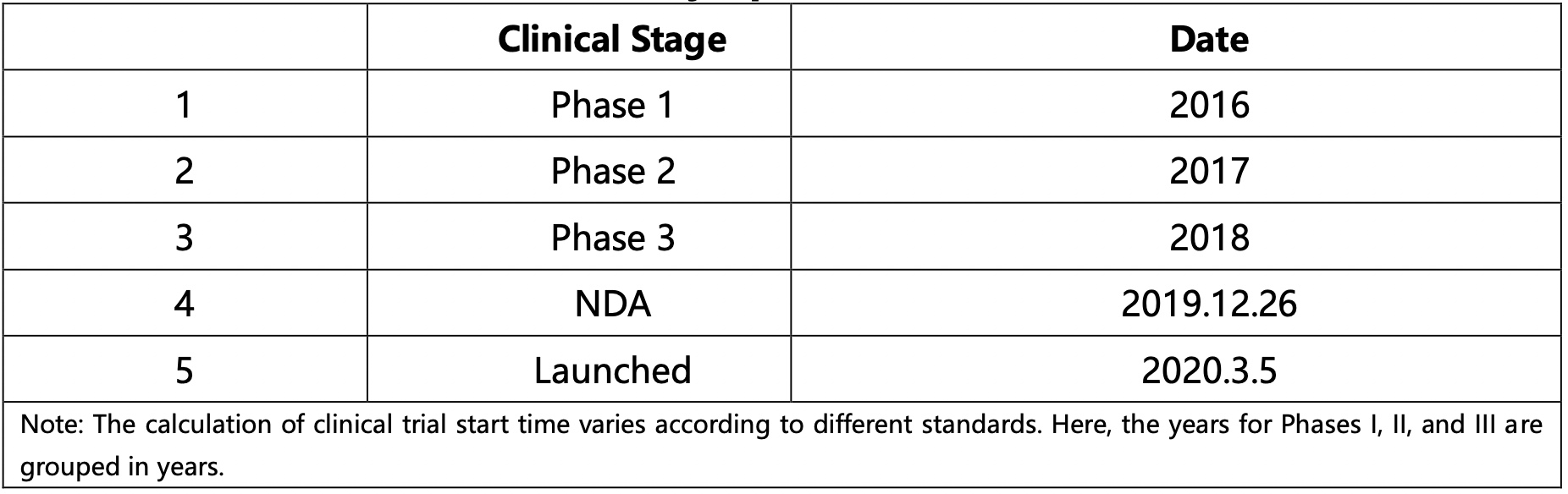
The Patsnap Synapse database also provides data on drug milestone events, helping users quickly grasp the above information
Preliminary Search of Tislelizumab-Related Patents and Core Substance Patents Through the Patsnap Synapse Database
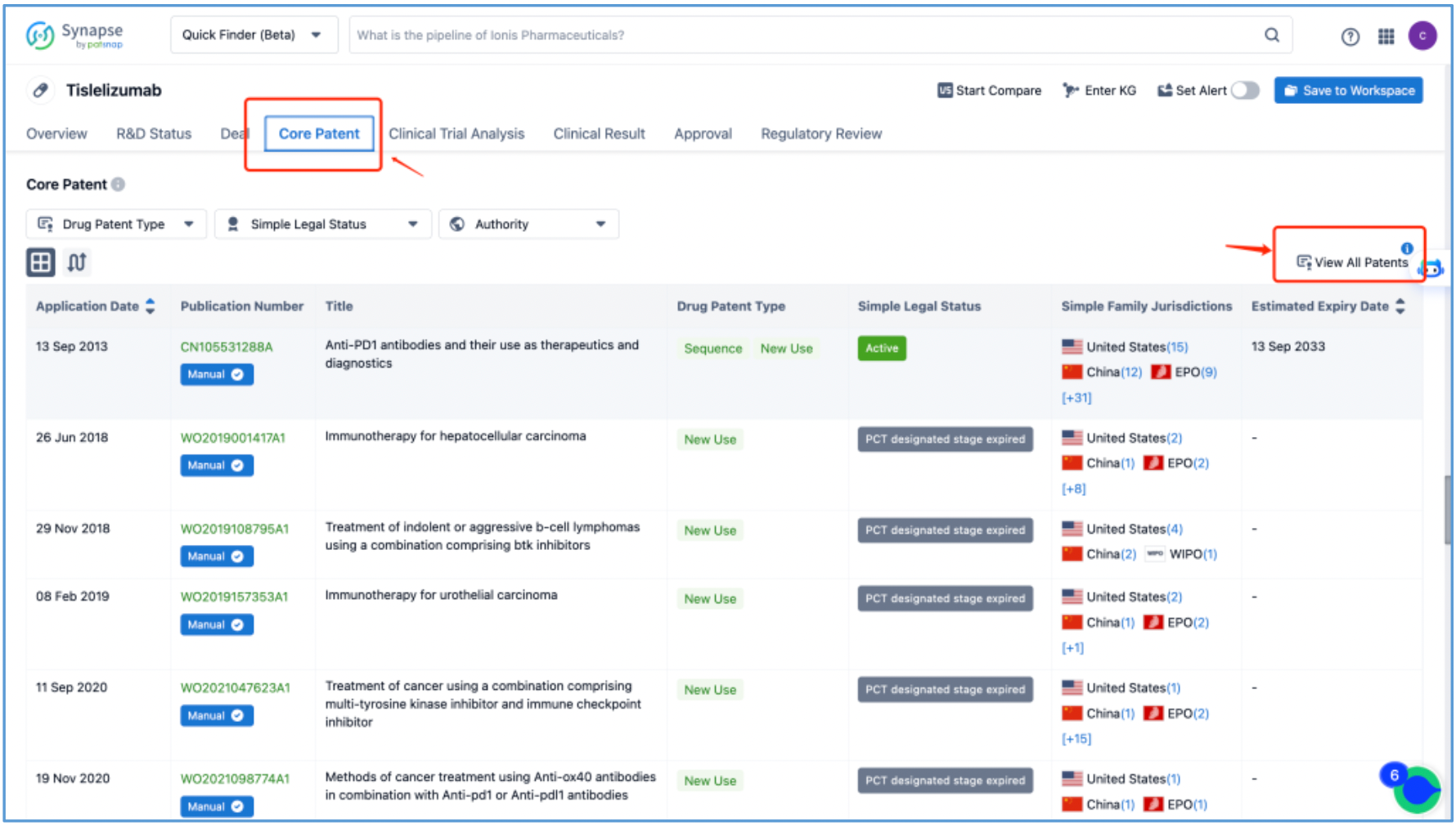
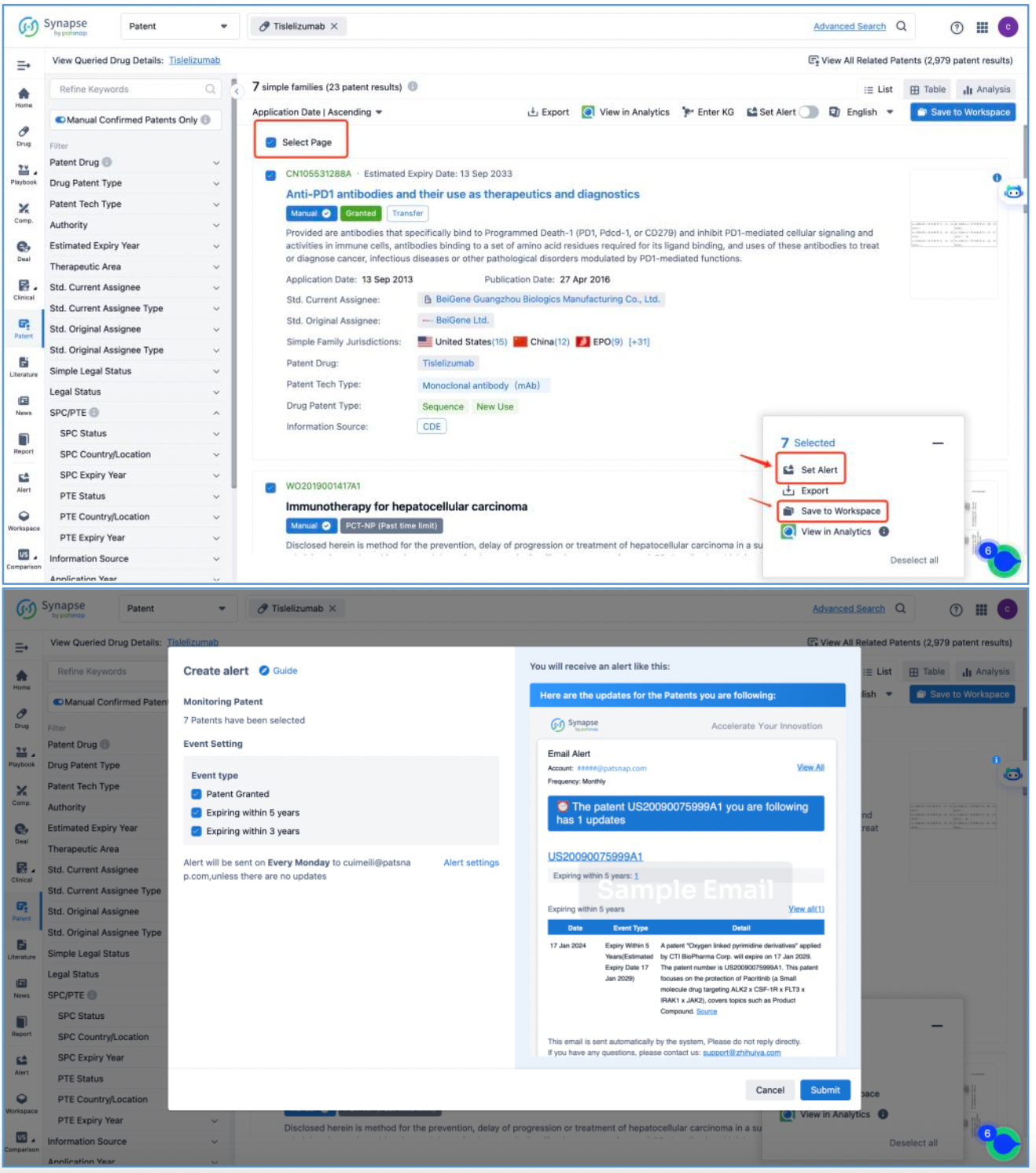
From the tislelizumab page, access the core patent interface and click "View All Patents" on the right to retrieve all patents associated with tislelizumab, amounting to 7 simple family patents. Select all desired patents and create an email alert to be notified of any updates to the core patent information. Then, save the patents to a new Workspace and name it‘tislelizumab.
Subsequently, application CN105531288A, "Anti-PD1 antibodies and their use as therapeutics and diagnostics", which is classified as Sequence and New Use patent types, is preliminarily identified as a core substance patent, prompting further analysis.
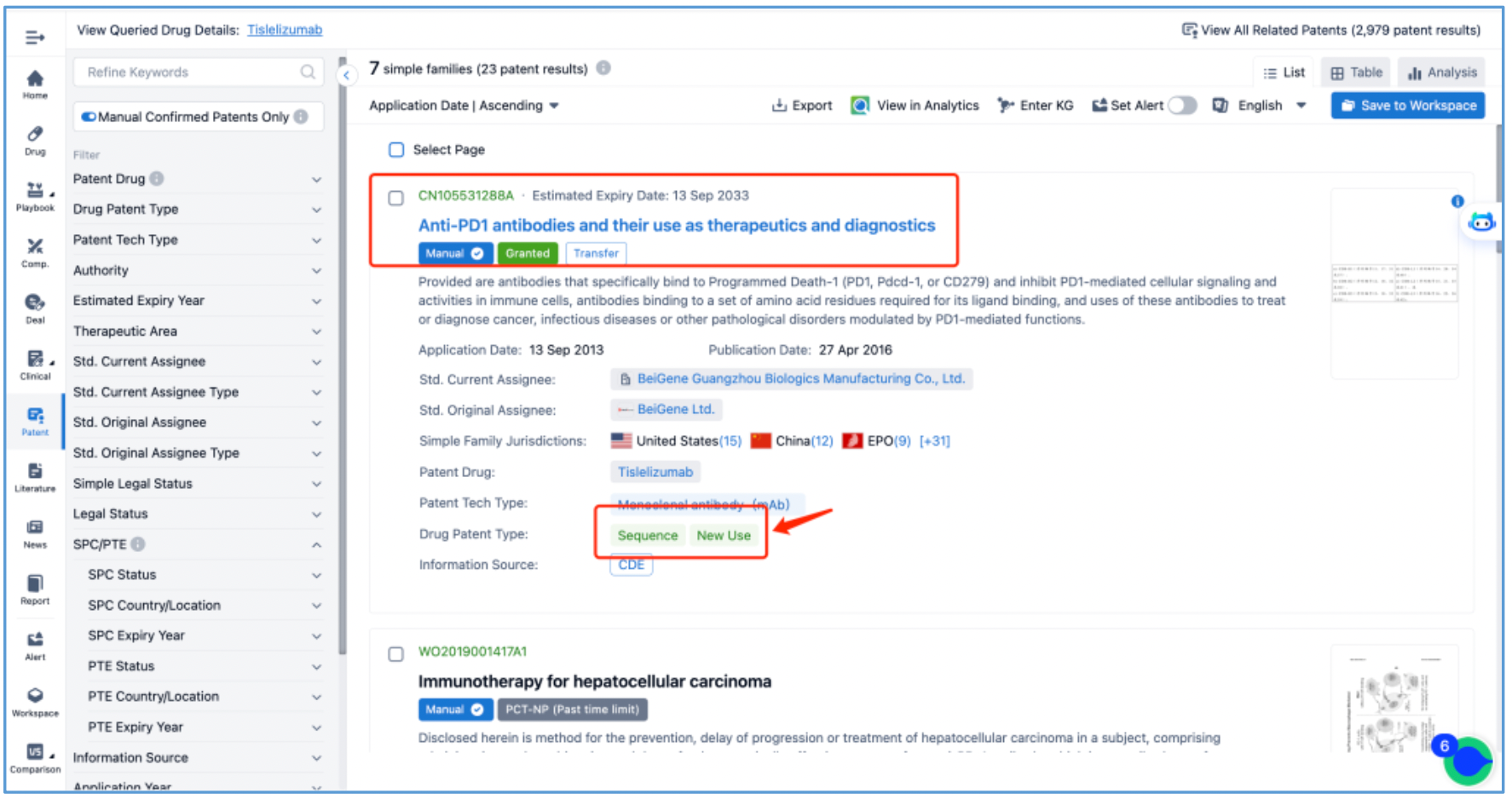
Analyzing Core Substance Patents to Ascertain the CDR Sequences Inherent to Tislelizumab
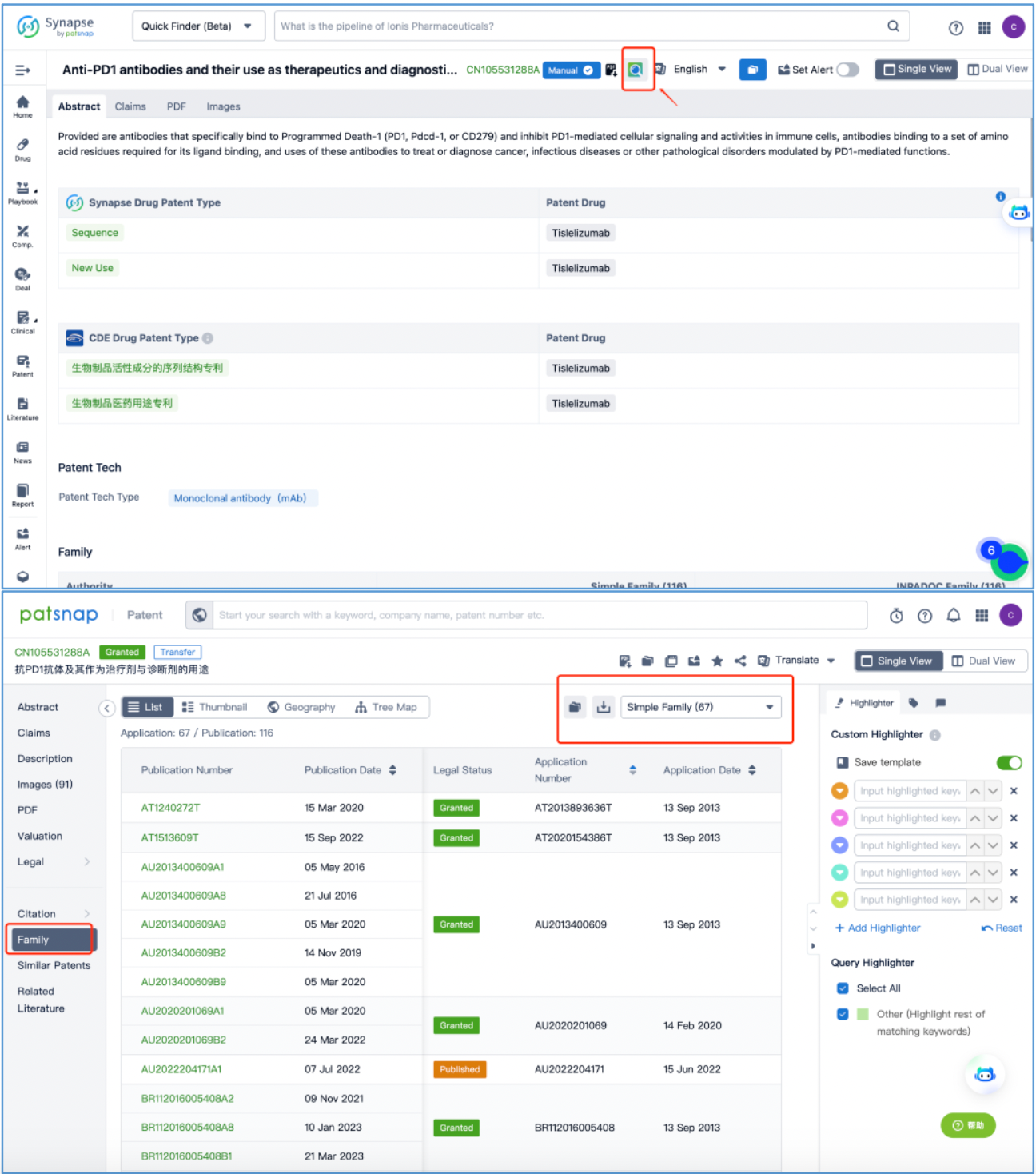
To access the patent details for CN105531288A, click on the patent and then proceed to the patent details page. For ease of subsequent patent analysis, directly select the "Patent Database" icon on this page to navigate to the Patsnap Analytics patent database and review the patent's detailed information.
Upon examination, it is initially determined that the core substance patent CN105531288A (Anti-PD1 antibodies and their use as therapeutics and diagnostics) comprises 67 simple family members and has been cited 39 times. This patent outlines antibodies that specifically target PD-1, impeding the cell signal transduction and activity mediated by PD1 in immune cells. It also defines a set of amino acid residues necessary for binding PD1 ligands and describes the application of these antibodies in treating or diagnosing cancers, infectious diseases, or other pathological conditions regulated by PD1-mediated functions.
From this assessment, it is evident that the core substance patent safeguards multiple antibody sequence CDRs. However, it does not explicitly specify the CDR sequences.
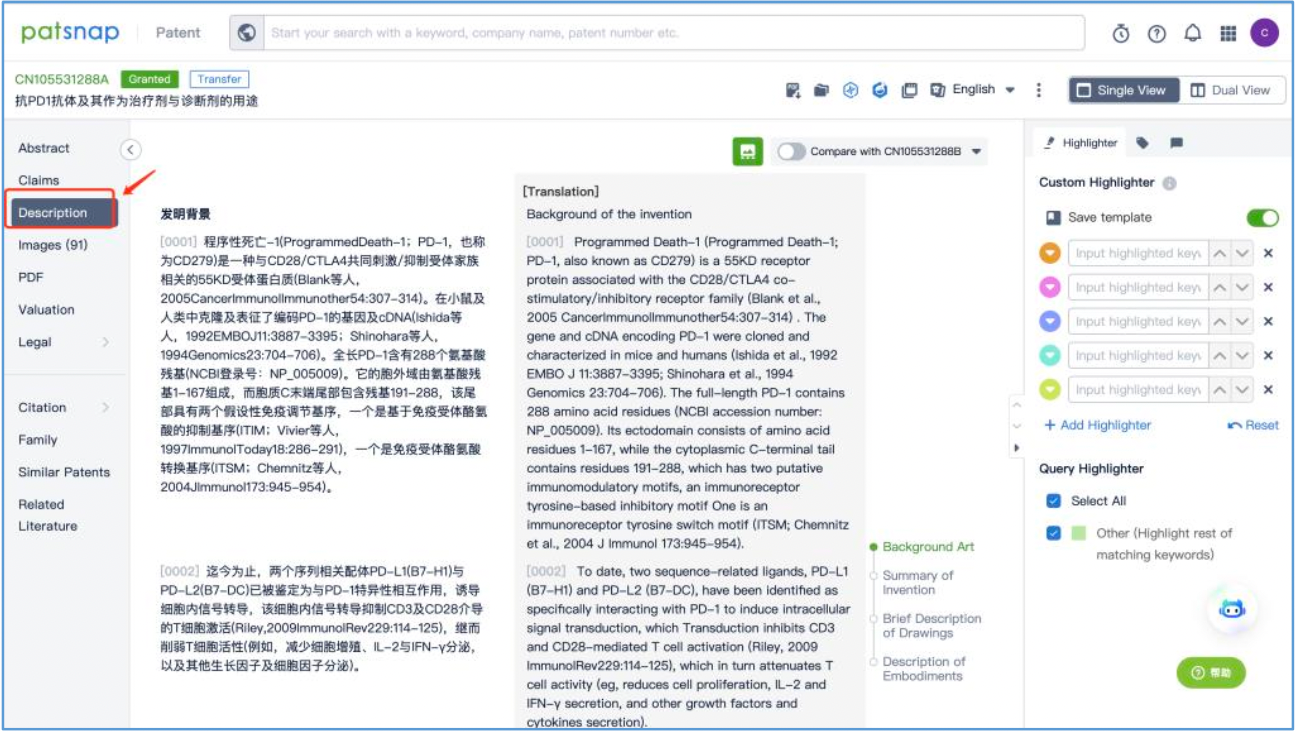
Subsequently, in the analysis phase, particular attention may be directed towards examining the pharmacological effects or ADCC efficacy data outlined in the "Description" section of the patents, such as the provided cytokine release and cytotoxicity assay data.
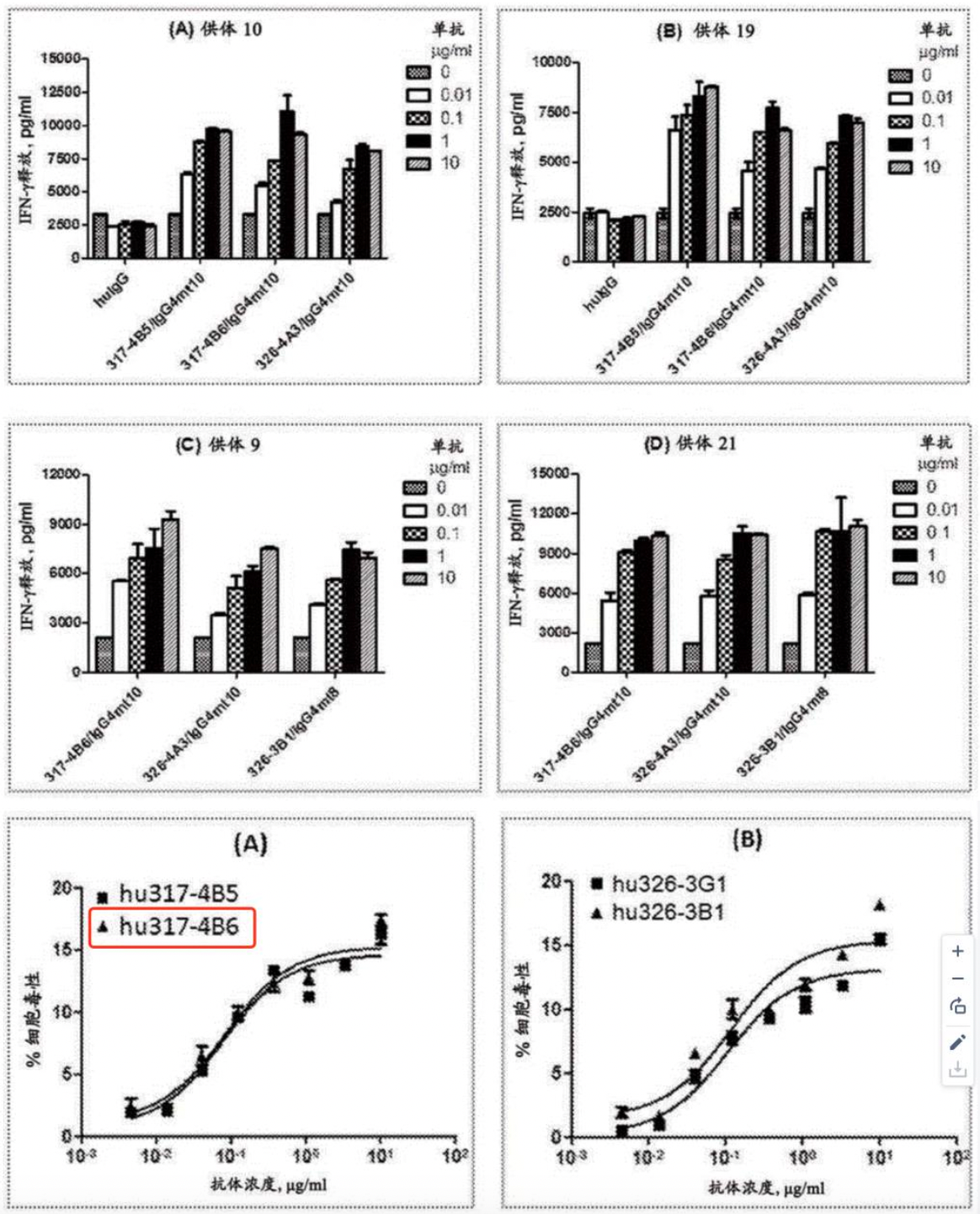
Following a thorough review of the patent and comprehensive analysis, it is concluded that the antibody identified as hu317-4B6 exhibits the most favorable efficacy among those mentioned in the patent. The CDR sequences corresponding to the antibody designated as hu317-4B6 are arranged as presented in the table below.
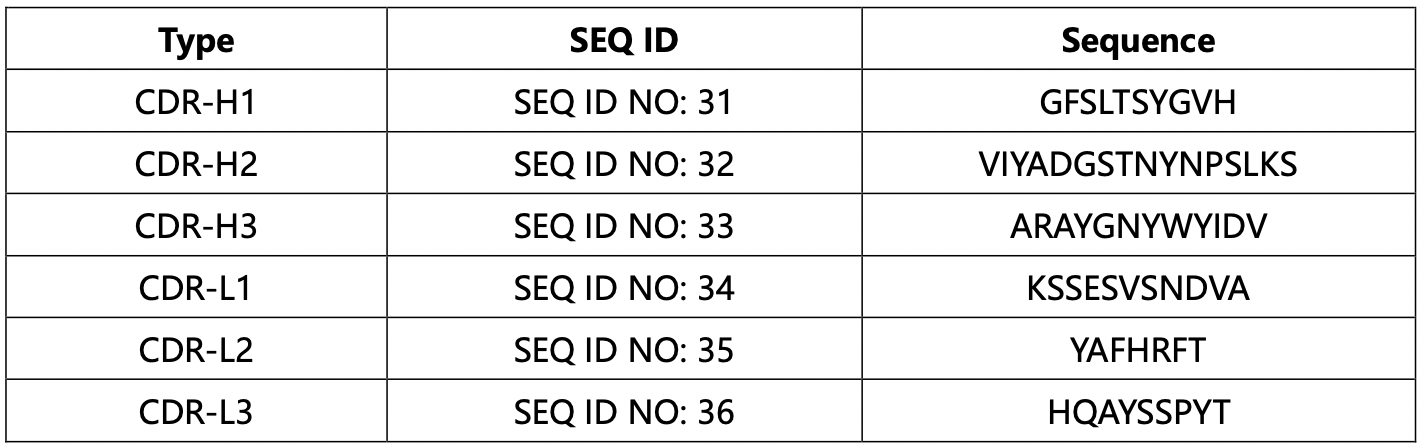
Finally, the above CDR sequences are validated using the VH and VL sequences collected in the basic information of tislelizumab in step 2.2. It is discovered that the heavy chain CDR sequences are within VH, and the light chain CDR sequences are within VL. Thus, we can conclude that the CDRs we identified are indeed the CDRs of the tislelizumab drug itself.
For more information, you can click the following article link:
BeiGene's Patent Research and Practical Operation Guide for Tislelizumab (3)
For more information, please click the image link below to access the full report.