BeiGene's Patent Research and Practical Operation Guide for Tislelizumab (1)
In recent years, the rise of biopharmaceuticals has surged. This rapidly growing sector, which employs living organisms or their derivatives in drug development, has transformed our approach to healthcare.
Monoclonal antibodies play a crucial role in the field of biopharmaceuticals, with Merck's PD-1 monoclonal antibody Keytruda emerging as a standout example of their impact. Keytruda has rapidly ascended to become the top-selling drug in the market, underscoring the significance of monoclonal antibodies in modern medicine.
In conducting patent research on monoclonal antibodies, the primary objective is to meticulously and concisely consolidate the patent data pertaining to the drugs under investigation. This process serves to furnish technical insights that are invaluable for infringement analysis, biosimilar drug development, investment, and financing decisions, as well as for guiding the research and development endeavors of pharmaceutical companies. By comprehensively summarizing the patent information, this research aims to provide a robust foundation for informed decision-making and strategic planning within the pharmaceutical industry.
In today's competitive landscape, the significance of a comprehensive monoclonal antibody drug patent research report cannot be overstated. This invaluable asset has emerged as the cornerstone of enterprise competitiveness, wielding a profound influence across various stages of drug development and commercialization.
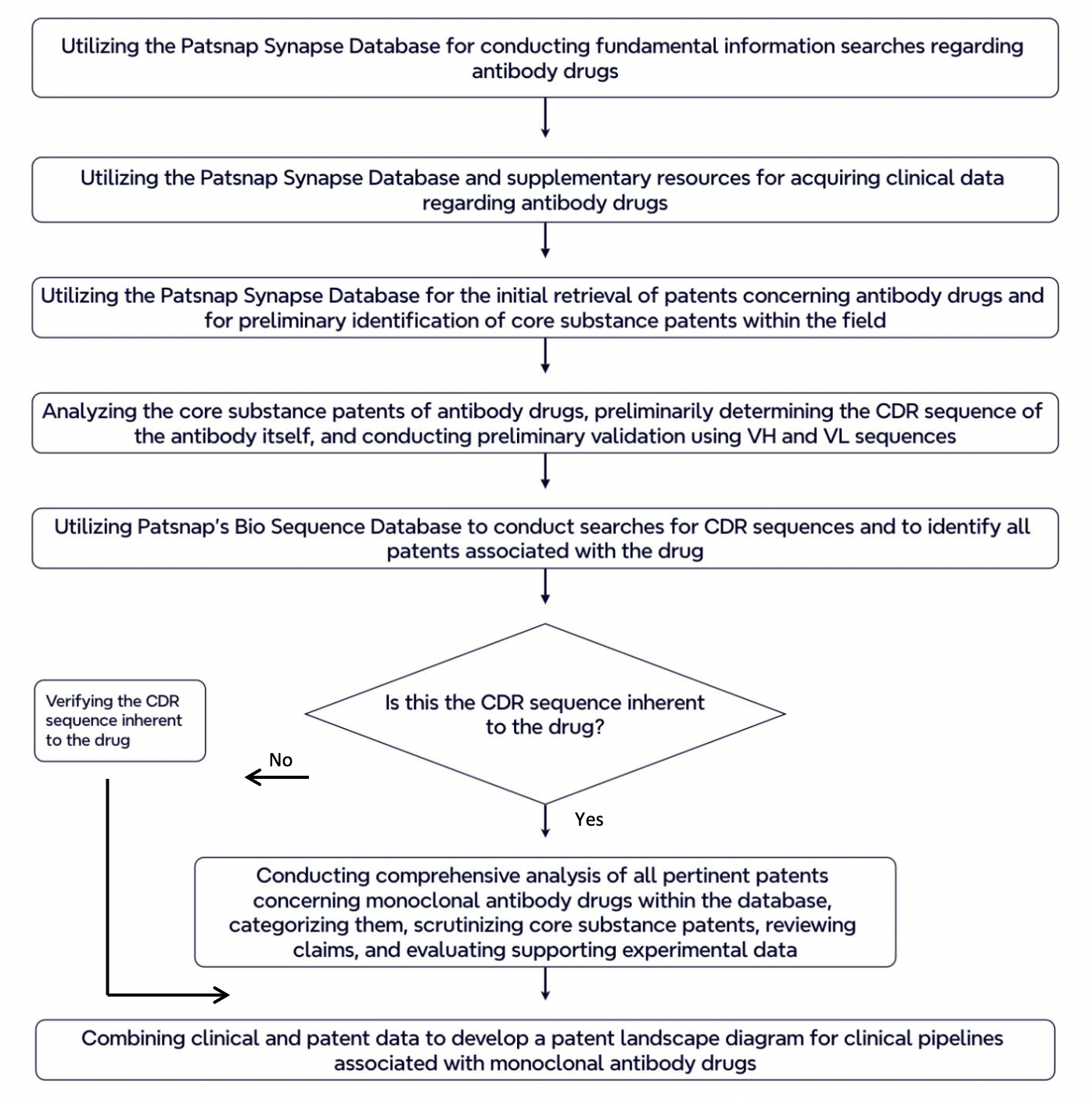
The process of patent research for monoclonal antibody drugs involves several key steps, including comprehensive patent searching and screening to identify relevant intellectual property, followed by in-depth analysis of drug clinical data. By carefully navigating each stage of this coordinated process, pharmaceutical companies can gain valuable insights that inform their strategic decision-making and ultimately contribute to the advancement of monoclonal antibody therapies. In the following sections, we will delve into each step of the patent research process, providing a detailed introduction to the complexities and considerations involved in this critical aspect of drug development.
Disclaimer:
The data presented in this report, primarily sourced from Patsnap Synapse, Bio, and Patsnap Analytics databases, may exhibit discrepancies due to data source leakage, variations in statistical periods, and divergent search methodologies. Therefore, it should be regarded as a reference only. This report is not responsible for any business losses resulting from the usage of this information.
Based on the characteristics of monoclonal antibody drugs, this report summarizes the steps of patent research for such drugs as follows. You can follow the process described below to effectively carry out your patent research on monoclonal antibody drugs.
For more information, please click the image link below to access the full report.