BioAtla gets FDA nod to start trials of BA3361, a CAB targeting Nectin-4 in diverse cancers
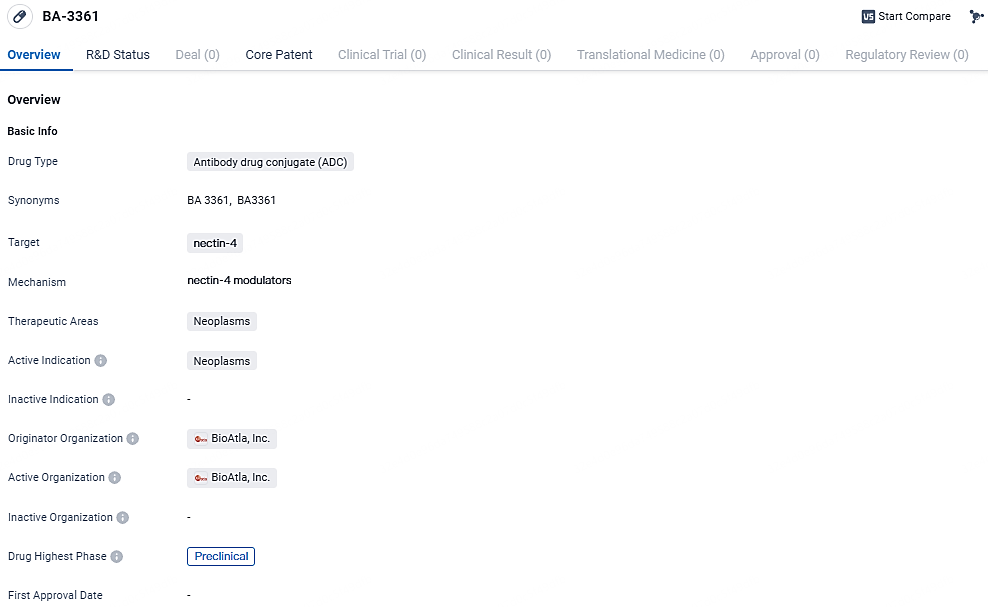
BioAtla, Inc., an international biotechnology firm engaged in clinical-stage research, specializes in creating Conditionally Active Biologic (CAB) antibody therapies aimed primarily at treating solid tumors. The company has recently gained approval from the U.S. Food and Drug Administration to proceed with clinical trials for its new investigational drug, BA3361 (CAB-Nectin-4) antibody drug conjugate, which targets various forms of tumors.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
ADC stands as an innovative therapy option with extensive potential in a variety of cancer types. The CAB technology is crafted to diminish both on-target, off-tumor and off-target, off-tumor toxicities. At the AACR Annual Meeting in April 2024, presentations highlighted the distinct capabilities of the anti-Nectin-4-ADC, showcasing its advanced next-generation carbohydrate linker system through both in vitro and in vivo studies.
The advanced NextGen linker system contributes to minimizing off-target, off-tumor toxic effects and has significantly enhanced serum stability and increased hydrophilicity, which boosts effectiveness. BioAtla’s CAB NextGen anti-Nectin-4-ADC exhibited complete tumor removal in multiple cell line derived xenograft models, demonstrated enhanced performance compared to an enfortumab vedotin analog in a pancreatic cancer model derived from a patient, and displayed decreased toxicity owing to CAB selectivity. BA3361 represents BioAtla’s pioneering glycoconjugate in the CAB-Nectin-4-ADC lineup.
“BioAtla is consistently presenting innovative candidates facilitated by CAB technology, highlighted by the recent FDA IND approval for our initial glycoconjugate, CAB-Nectin-4-ADC, BA3361,” stated Jay M. Short, Ph.D., Chairman, CEO and co-founder of BioAtla, Inc. “The prospect of targeting Nectin-4 is thrilling, especially as our combination of CAB technology and the NextGen linker system seeks to optimize the therapeutic index and broaden uses across several cancer types.”
BA3361, CAB-Nectin4-ADC, serves as a conditionally active and reversible antibody drug conjugate aimed at Nectin4, a protein facilitating cell-cell adhesion which is predominantly expressed in various human tumors. The binding sites of BA3361 specific to Nectin4 are engineered for optimal interaction within the tumor environment while minimizing activity under normal conditions.
BA3361 is the inaugural molecule integrating one of BioAtla’s NextGen ADC linkers, delivering improved stability and selective release of the drug within tumors. The efficacy of BA3361 has been proven superior in patient-derived xenograft models of pancreatic cancer.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
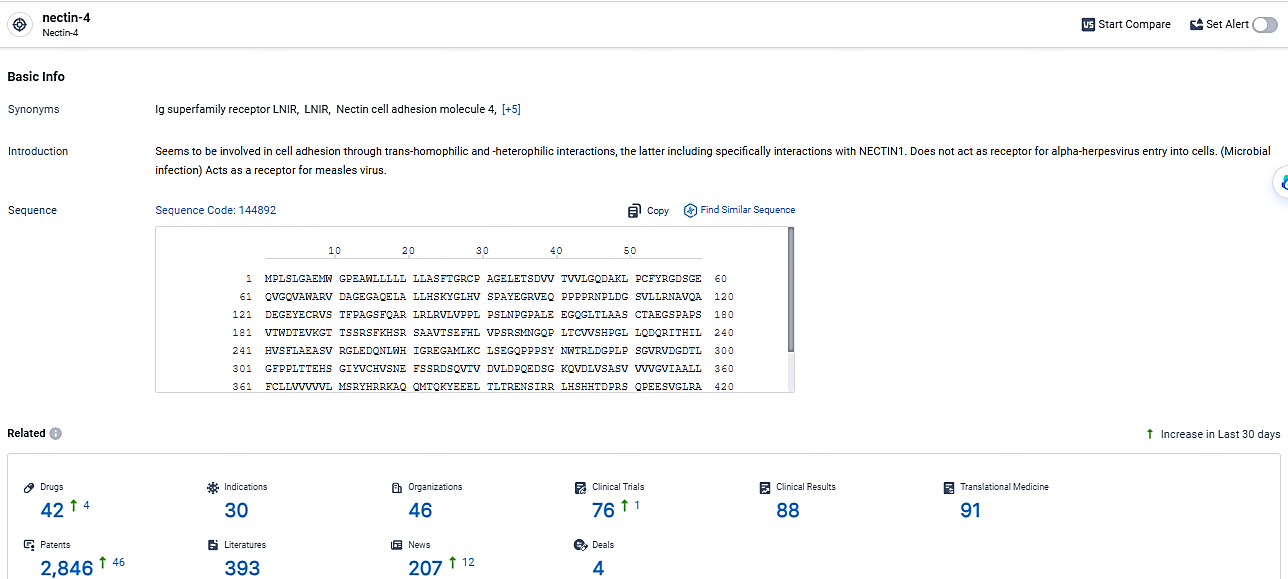
According to the data provided by the Synapse Database, As of May 7, 2024, there are 42 investigational drugs for the nectin-4 target, including 30 indications, 46 R&D institutions involved, with related clinical trials reaching 76, and as many as 2846 patents.
BA-3361 is an antibody drug conjugate that targets nectin-4 and is being developed by BioAtla, Inc. for the treatment of neoplasms. It is currently in the preclinical phase of development, where its safety and efficacy are being evaluated. Further research and clinical trials will be necessary to determine the potential of BA-3361 as a therapeutic option for cancer patients.