What ADC-related progress will Kelun Botai Biomedicine reveal at the ASCO conference?
At the upcoming 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Kelun Botai Biomedicine will disclose the research progress of SKB264. The SKB264 disclosed this time is China's first domestically produced TROP-2 ADC, with a high DAR value (7.4). The toxin selected is a novel topoisomerase I (TOPO1) inhibitor derived from belotecan, exhibiting moderate cytotoxicity. The proprietary Kthiol design strategy utilizes a novel carbonate linker to selectively release cytotoxic payloads in the acidic tumor microenvironment. It has been granted four breakthrough therapy designations by the CDE for advanced or metastatic triple-negative breast cancer (TNBC), advanced or metastatic EGFR-mutant non-small cell lung cancer (NSCLC), and advanced, recurrent, or metastatic PD-L1-negative TNBC. In December 2023, the marketing application for SKB264 was accepted and included in the priority review for the indication of advanced TNBC.
Kelun Botai and its partner Merck are currently working diligently to explore the potential of SKB264 in the treatment of lung cancer. To date, they have initiated seven global phase III clinical trials around SKB264 (MK-2870), including:
·For EGFR mutation-positive NSCLC progressing after immunotherapy and chemotherapy, MK-2870-004, with 556 participants;
·Advanced endometrial cancer, MK-2870-005, with 710 participants;
·PD-L1 highly expressing (TPS≥50%) metastatic NSCLC, MK-2870-007, with 614 participants;
·EGFR mutation-positive NSCLC progressing after immunotherapy, MK-2870-009, with 520 participants;
·A global phase III clinical trial for monotherapy or in combination with Keytruda in locally advanced or metastatic HR+/HER2- breast cancer, MK-2870-010, with 1,200 participants;
·SKB264 (MK-2870) + Keytruda vs Keytruda for adjuvant therapy in resectable stage II-IIIB (N2) NSCLC patients who did not achieve pathological complete remission after neoadjuvant Keytruda and platinum-based doublet chemotherapy, MK-2870-019, with 780 participants;
·Recurrent or metastatic gastric cancer, MK-2870-015, with 450 participants.
Previously disclosed global phase 1/2 trial preliminary clinical data demonstrated encouraging efficacy and potential good safety of SKB264 in a variety of heavily pre-treated advanced solid tumors. Among 59 treated metastatic TNBC (mTNBC) patients, the objective response rate (ORR) was 42.4%, disease control rate (DCR) was 76.3%, median duration of response (mDoR) was 11.5 months, median progression-free survival (mPFS) was 5.7 months, and median overall survival (mOS) reached 16.8 months, with 12-month and 24-month OS rates at 65% and 39.5% respectively. Nearly 40% of patients survived more than two years. In the subgroup of high TROP2 expressers (H-score>200, n=32), the ORR was 53.1%, mDoR was 11.1 months, mPFS was 5.8 months, mOS was not reached, and the 12-month and 24-month OS rates were 65.3% and 57.3%, respectively. Non-head-to-head data comparison showed superior ORR and mPFS compared to Trodelvy and DS-1062. While demonstrating excellent efficacy, the overall safety profile was generally good with a rate of adverse events grade greater than 3 occurring in 59.3% of cases, lower than Trodelvy (64%).
Additionally, phase I/II data for the treatment of NSCLC showed: In the EGFR wild-type subgroup, the ORR was 26%, DCR was 89%, and mPFS was 5.3 months. In the TKI-resistant EGFR-mutant NSCLC subgroup, the ORR was 60%, DCR was 100%, and mPFS was an impressive 11.1 months. SKB264 exhibited particularly outstanding efficacy and good safety in the treatment of TKI-resistant EGFR-mutant NSCLC, with no observed side effect-related discontinuations or deaths.
Kelun Botai is a biopharmaceutical company focused on the innovation, development, manufacture, and commercialization of drugs in oncology, immunotherapy, and other therapeutic areas. The company has accumulated over ten years of experience in ADC development to address medical needs in specific disease areas such as oncology, autoimmune diseases, and metabolic diseases.
How to search for and analyze the development progress of ADC pharmaceuticals?
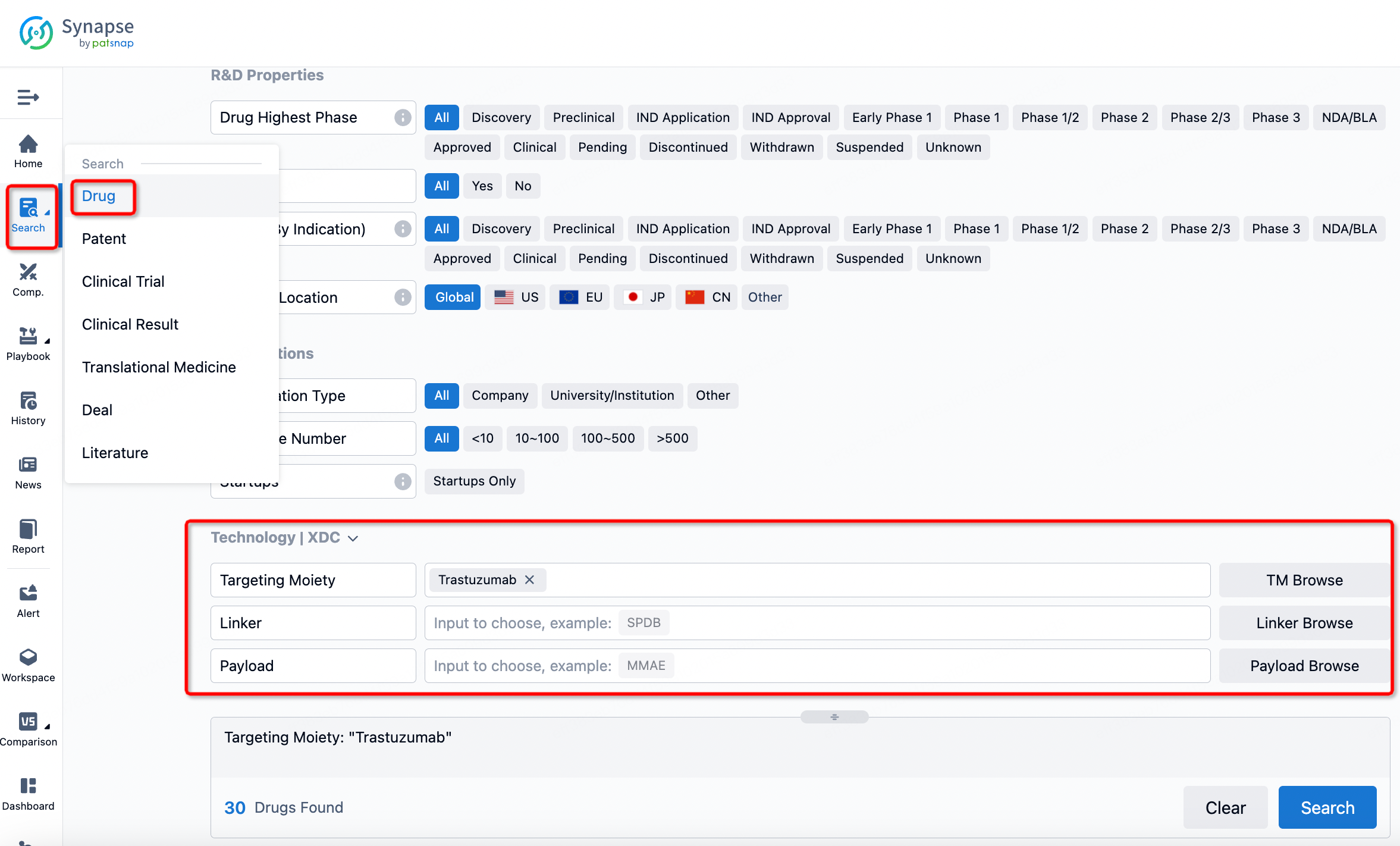
If you want to learn about the latest developments in ADC drugs, you can use the drug search module of the Synapse database. This module supports searching for ADC drugs by classification through Targeting Moiety, Linker, and Payload.
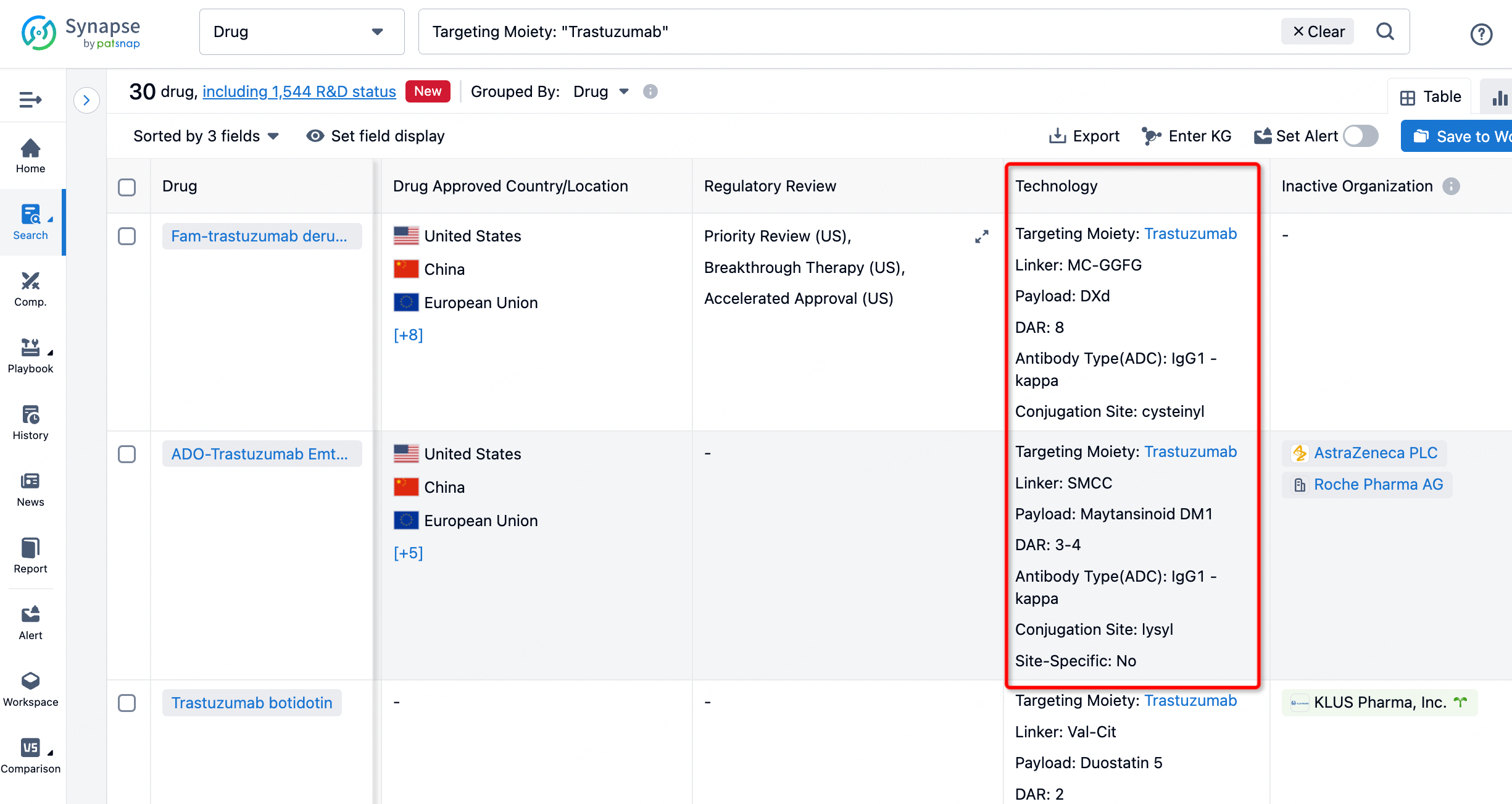
On the search results page, you can easily review information related to the ADC's technical category of the drugs.
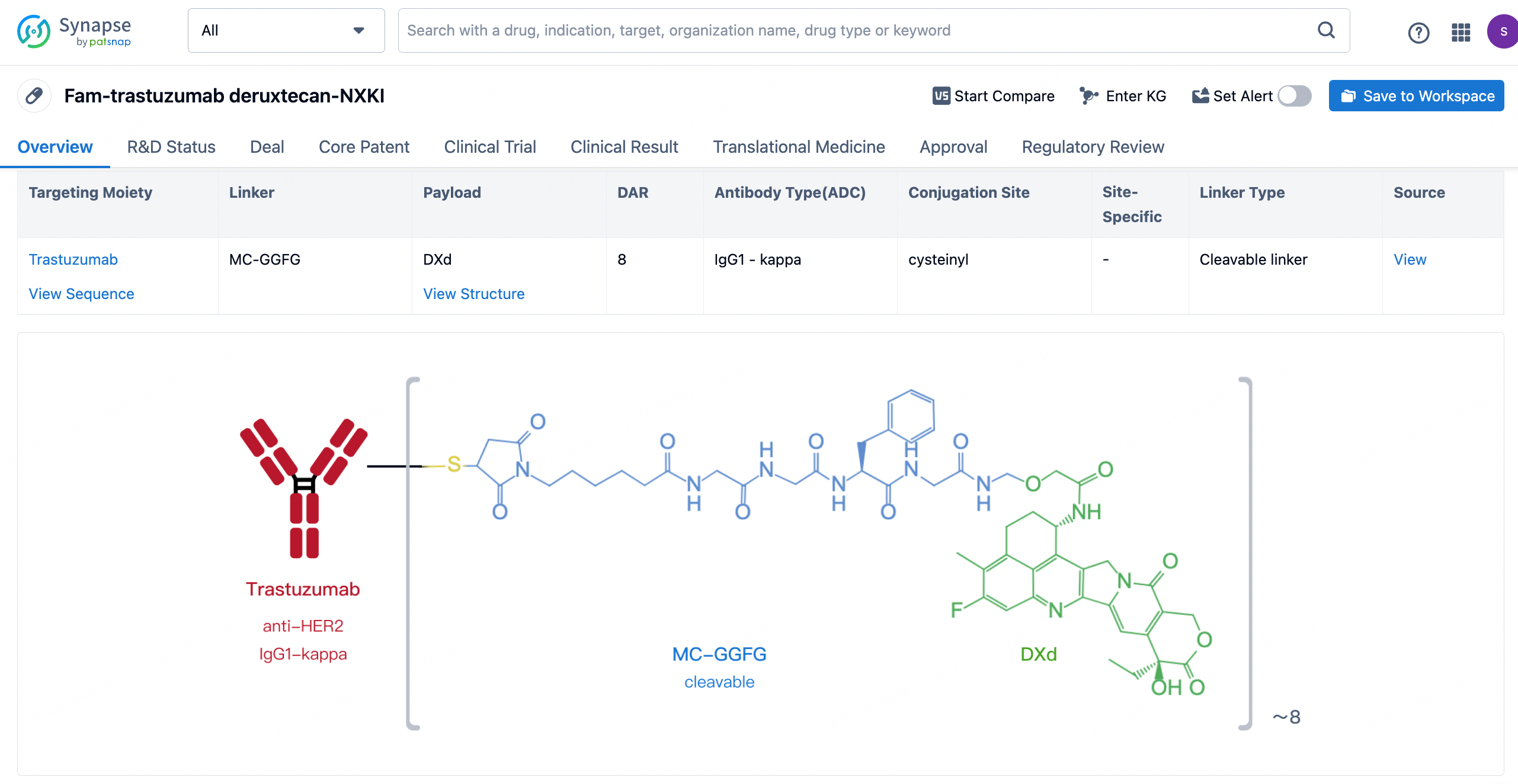
After clicking to enter the drug details page, you can also effortlessly obtain structural information about the ADC drug.
Click on the image below to embark on a brand new journey of drug discovery!