Biogen Presents Positive Phase 2 Results for Felzartamab in IgA Nephropathy at ASN Kidney Week 2024
Biogen Inc. has released comprehensive findings from the Phase 2 IGNAZ trial that assessed felzartamab, a novel anti-CD38 monoclonal antibody, in patients with IgA nephropathy. The findings demonstrated significant decreases in proteinuria, maintenance of kidney function, and a lasting treatment effect lasting more than 18 months after administering the final dose of felzartamab. These complete results were presented orally at Kidney Week 2024, the annual meeting of the American Society of Nephrology, held in San Diego, California.
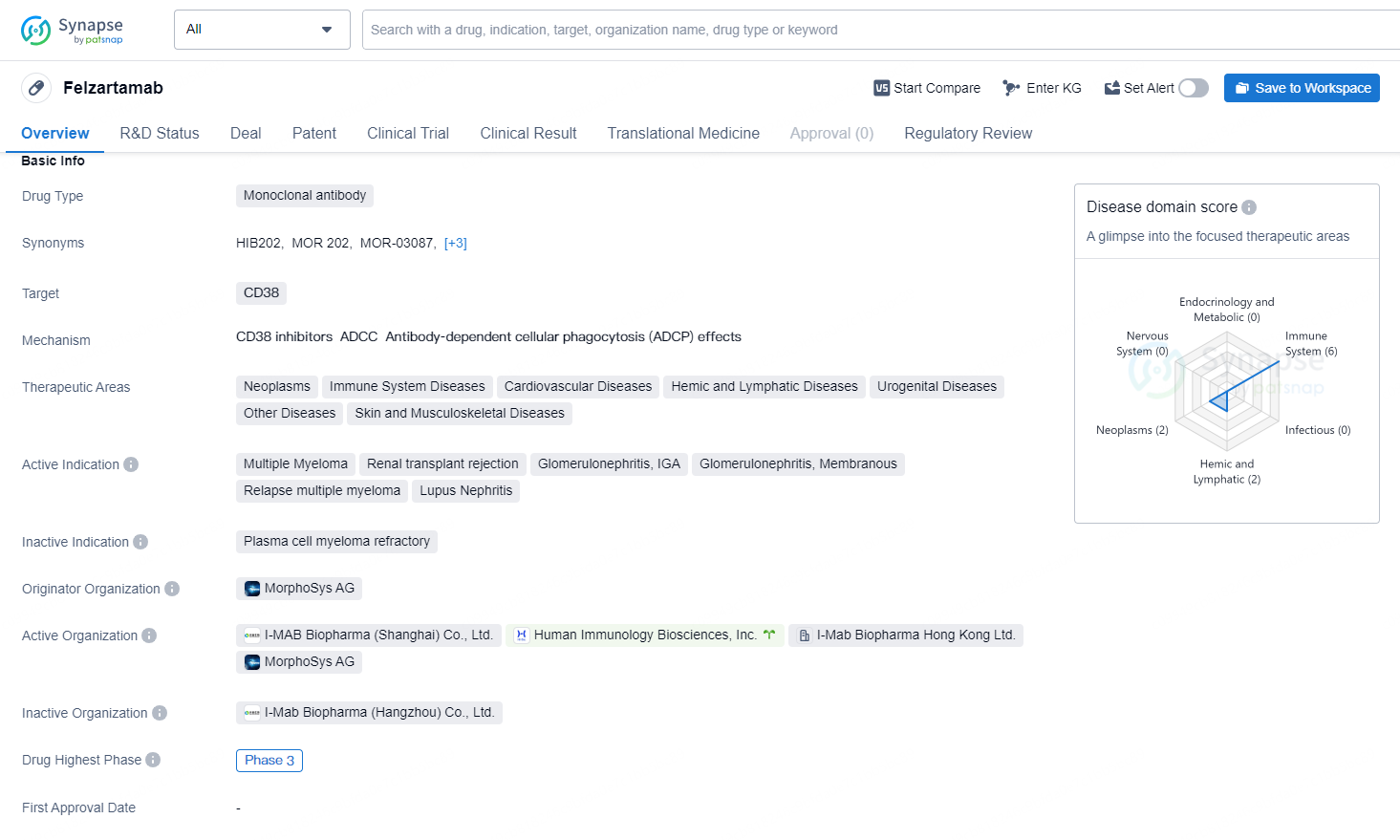
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
“The complete results of the IGNAZ Study reaffirm our interim findings, showing a reduction in proteinuria, stabilization of kidney function, and sustained treatment effect more than 18 months after the last dose of felzartamab,” said Jonathan Barratt, MD, PhD, FRCP, Mayer Professor of Renal Medicine at the University of Leicester. “This is promising news for patients and supports the potential of felzartamab to be a meaningful treatment option for people living with IgA nephropathy, a leading cause of chronic kidney disease.”
Further analysis revealed that felzartamab administration resulted in selective and durable reductions in IgA antibody levels, while IgG and IgM levels recovered to baseline 3 months off-treatment. This selective reduction may offer maintenance of significant immune functions essential for infection protection. Overall, administration of felzartamab was generally well tolerated with a safety profile consistent with prior studies.
“We are encouraged by the overall results of the IGNAZ study, especially given the significant unmet medical need for additional treatments to address high-risk IgA nephropathy,” said Uptal Patel, M.D., Head of Development, HI-Bio at Biogen. “We are grateful to all the participants, investigators and study staff who contributed to this study, whose findings will help us continue to evaluate felzartamab’s role in preserving kidney function as we plan for Phase 3.”
Felzartamab is an investigational therapeutic human monoclonal antibody directed against CD38, a protein expressed on mature plasma cells. Felzartamab is a potential first-in-class therapeutic candidate with promise as a pipeline-in-a-product across a range of immune-mediated diseases. Felzartamab has been shown in clinical studies to selectively deplete CD38+ plasma cells, which may allow applications that ultimately improve clinical outcomes in a broad range of diseases driven by pathogenic antibodies.
Felzartamab was originally developed by MorphoSys AG for multiple myeloma. Human Immunology Biosciences exclusively licensed the rights to develop and commercialize felzartamab across all indications in all countries and territories excluding China. Biogen acquired HI-Bio in July 2024.
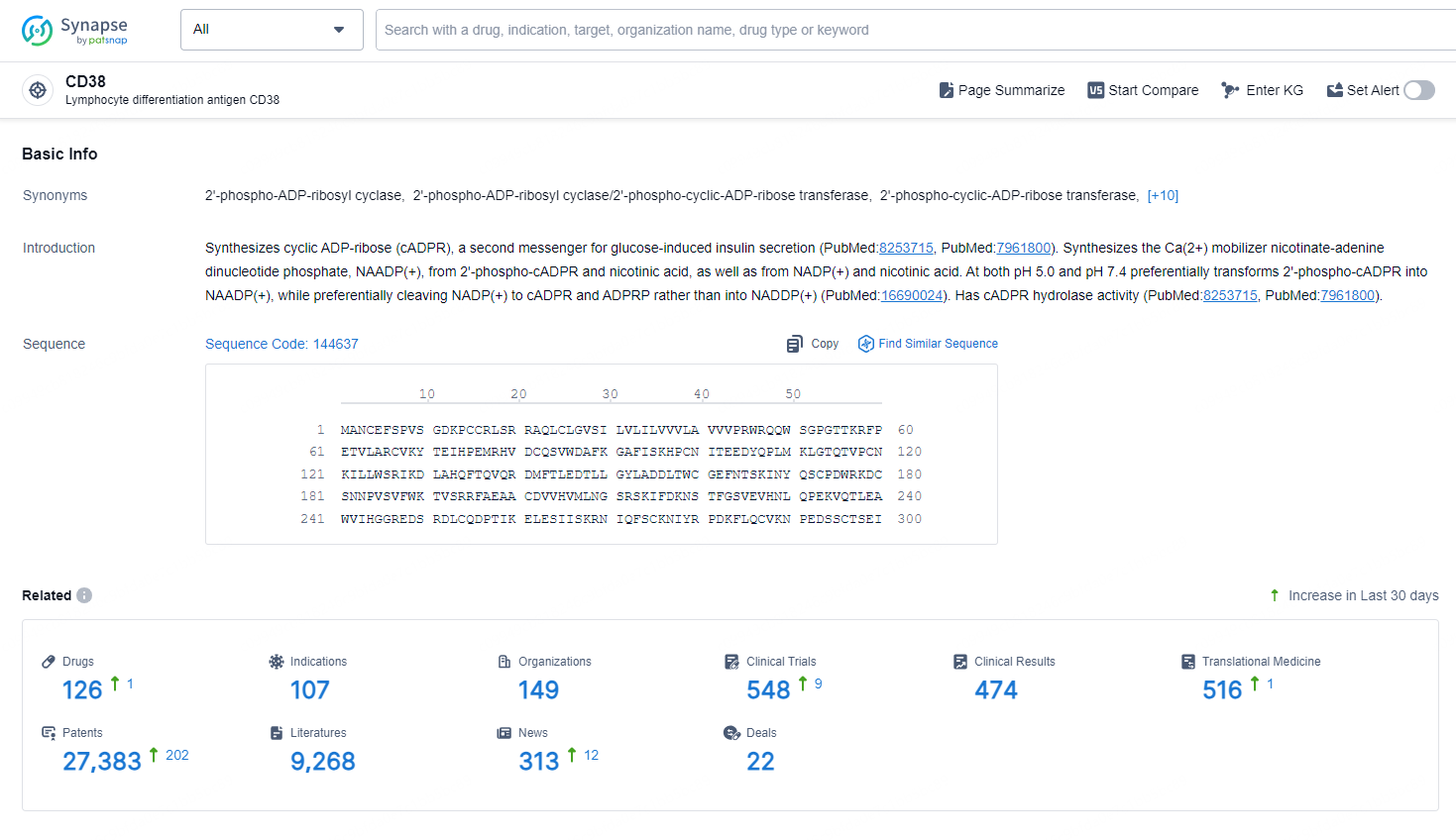
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of October 30, 2024, there are 126 investigational drugs for the CD38 target, including 107 indications, 149 R&D institutions involved, with related clinical trial reaching 548, and as many as 27383 patents.
Felzartamab has shown significant potential as a therapeutic option for a diverse range of diseases, and its progression to advanced clinical development stages and regulatory designations reflect its importance in the pharmaceutical industry. The regulatory designations of orphan drug and breakthrough therapy further underscore the potential significance of Felzartamab in addressing unmet medical needs and in providing new treatment options for patients with the specified diseases.