Zymeworks Showcases Latest ADC Preclinical Data at EORTC-NCI-AACR Conference
Zymeworks Inc. (Nasdaq: ZYME), a biotechnology firm in the clinical phase, is focused on creating a variety of innovative, multifunctional biotherapeutics aimed at enhancing treatment standards for challenging diseases. The company recently revealed new preclinical findings regarding its antibody-drug conjugate (ADC) candidates, ZW220 and ZW251, which were presented at the European Organisation for Research and Treatment of Cancer-National Cancer Institute-American Association for Cancer Research (EORTC-NCI-AACR) Conference held in Barcelona from October 23 to 25, 2024.
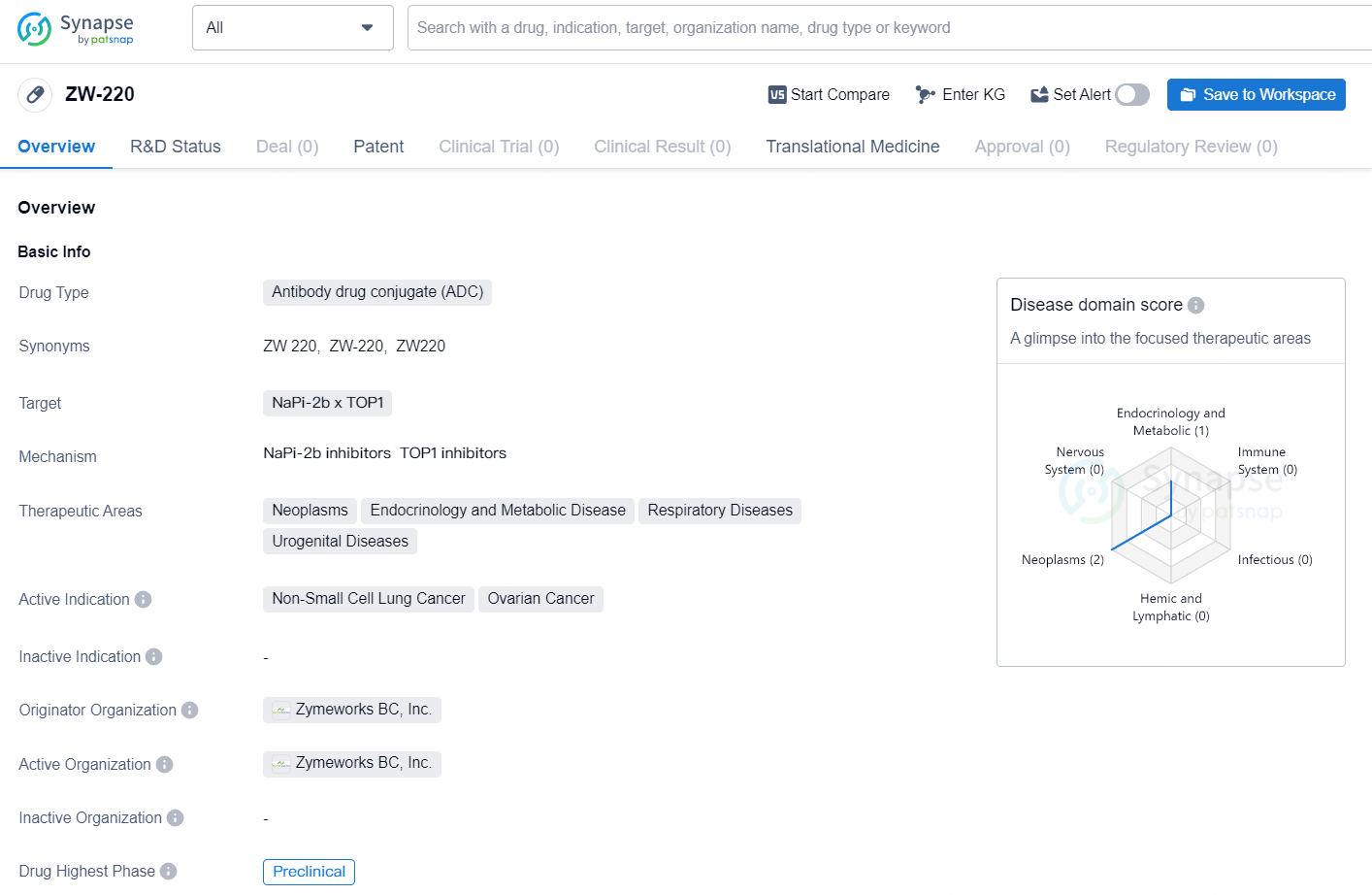
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"We are excited to share the preclinical findings for ZW220 and ZW251 during the EORTC-NCI-AACR Conference," stated Paul Moore, Ph.D., Chief Scientific Officer at Zymeworks. "These uniquely developed molecules, which utilize our proprietary payload ZD06519, exhibit considerable promise in addressing pressing needs within oncology. The preclinical findings unveiled this week reinforce our confidence that these projects could enhance treatment alternatives beyond the current standards, providing renewed optimism for patients facing difficult cancers where therapeutic advancements have been inadequate.”
An oral presentation titled “ZW220, a NaPi2b-targeting topoisomerase I inhibitor antibody-drug conjugate, displays compelling preclinical efficacy in NSCLC, ovarian, and uterine cancer models with a favorable toxicity profile in non-human primates” underscores preclinical data that strengthens the case for an IND application in the early part of 2025.
The findings reveal that ZW220 may offer advantages over previous NaPi2b ADCs in terms of effectiveness, tolerance, and payload mechanism. ZW220 utilizes a novel, moderately potent TOPO1i payload that demonstrates robust bystander activity, which is advantageous for tumors with low and variable NaPi2b expression levels. We believe it provides a distinct safety profile compared to other ADCs currently undergoing clinical evaluation, showing high tolerance in animal trials with a maximum tolerated dose (MTD) of ≥90 mg/kg in non-human primates and ≥200 mg/kg in rats, indicating a potential for elevated doses in humans. The low drug-to-antibody ratio (DAR) and moderate stability of the antibody-linker construct afford an optimal combination of stability, tolerability, and anti-tumor activity while likely reducing antibody-related toxicities. The efficient internalizing nature of ZW220’s antibody enhances cellular transport and tissue infiltration, which may boost anti-tumor efficacy even in cases of reduced NaPi2b expression.
A poster titled “ZW251, an innovative glypican-3-targeting antibody-drug conjugate equipped with a topoisomerase I inhibitor payload, exhibits compelling preclinical activity in hepatocellular carcinoma models” showcases preclinical findings that further bolster the rationale for an IND submission anticipated in the latter half of 2025.
The results indicate that ZW251 has potential as a novel therapeutic option for patients, potentially providing enhancements over the existing standard of care (SOC). With strong anti-tumor activity observed across a wide array of hepatocellular carcinoma (HCC) models, including those with lower and heterogeneous GPC3 expression, ZW251 features a DAR of four, which offers a balance between tolerability and extensive anti-tumor efficacy. In non-human primate studies, ZW251 demonstrated notable tolerability at doses up to 120 mg/kg. As an ADC, ZW251 offers versatile treatment approaches, possibly functioning as either monotherapy or in conjunction with current SOC therapies.
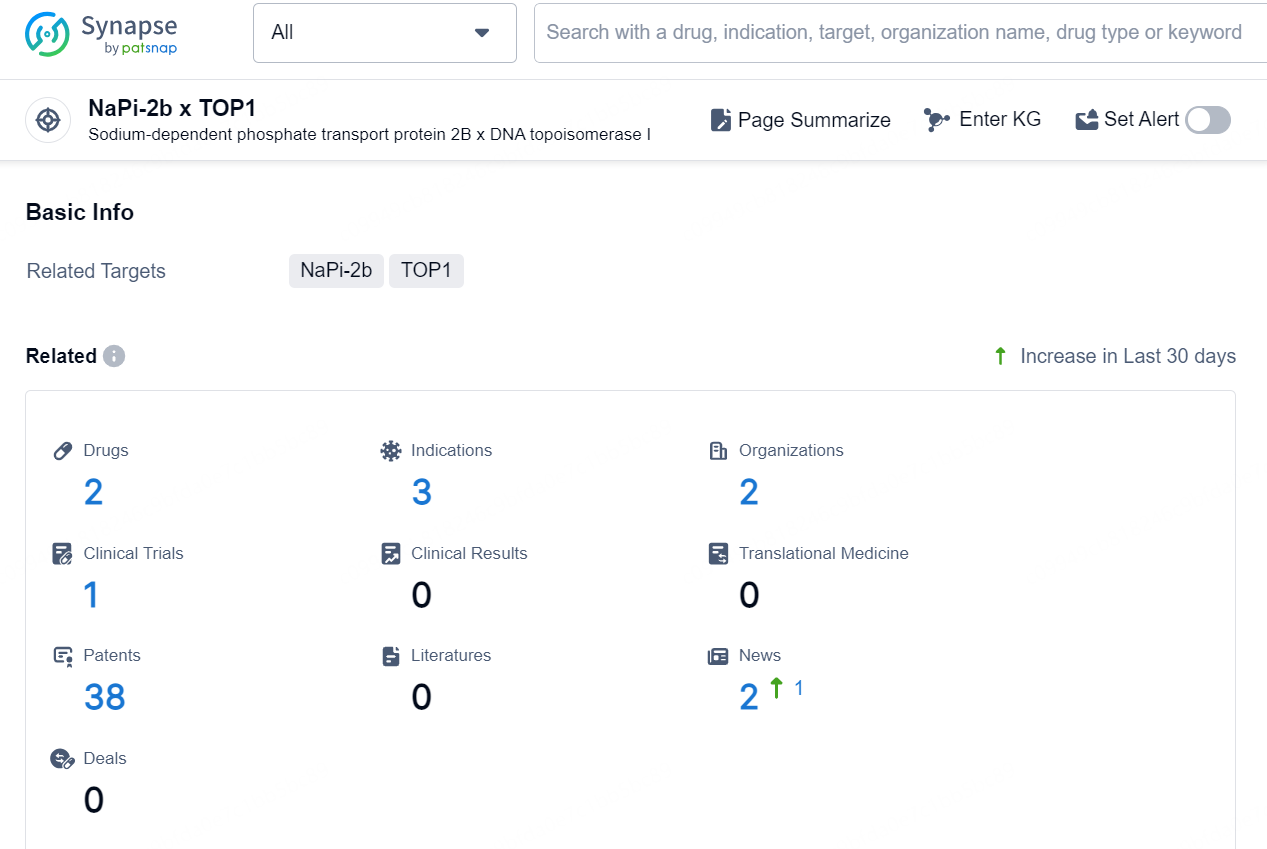
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 29, 2024, there are 2 investigational drugs for the NaPi-2b x TOP1 targets, including 3 indications, 2 R&D institutions involved, with related clinical trial reaching 1, and as many as 38 patents.
The drug ZW-220 is an antibody drug conjugate (ADC) targeting NaPi-2b x TOP1. It is currently being developed to treat a range of therapeutic areas including neoplasms, endocrinology and metabolic disease, respiratory diseases, and urogenital diseases. Its active indication is for the treatment of non-small cell lung cancer and ovarian cancer.