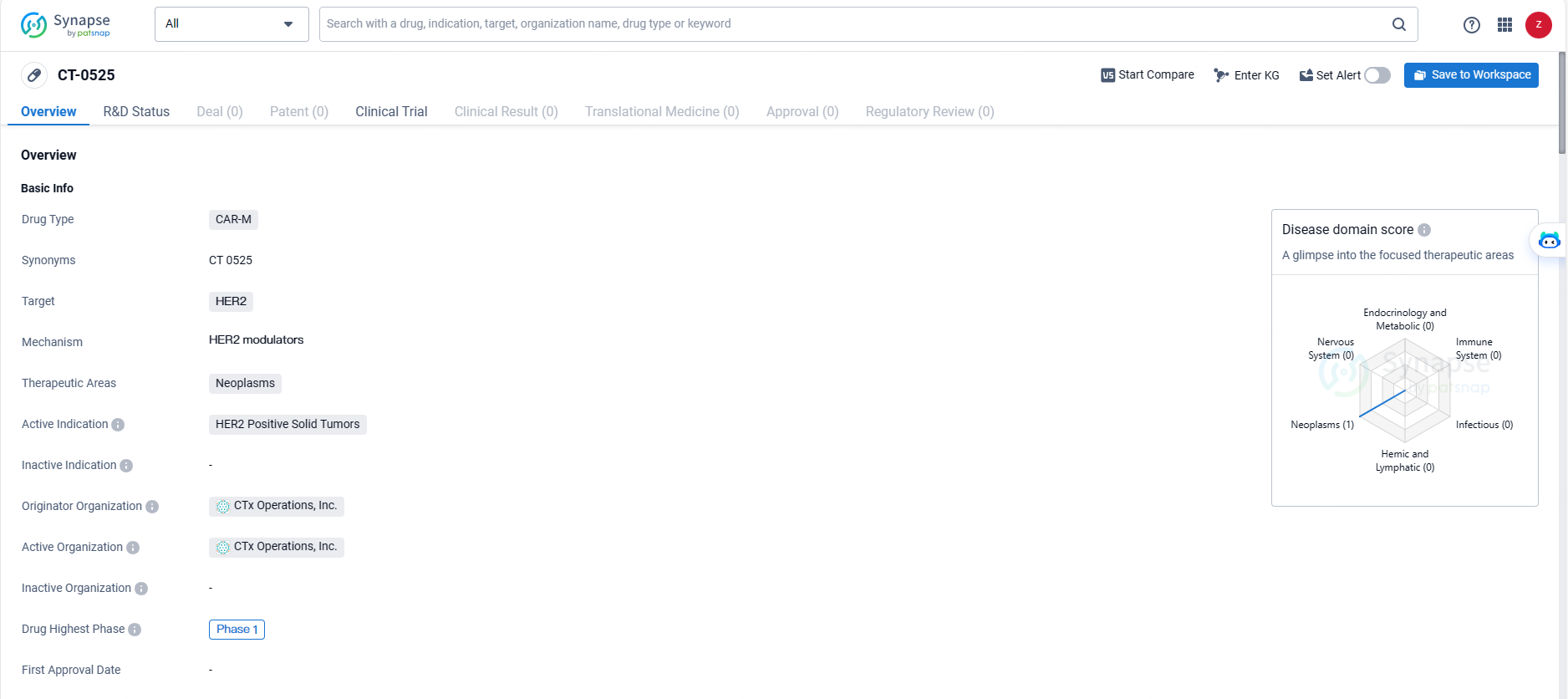
Carisma Therapeutics Begins Phase 1 Trial of CT-0525 HER2 CAR-Monocyte Therapy
Carisma Therapeutics Inc., a biopharmaceutical company in the clinical stage dedicated to the development of novel immunotherapies, disclosed that the initial participant has been treated in its Phase 1 clinical trial. This trial investigates CT-0525, a gene-modified autologous chimeric antigen receptor-monocyte (CAR-M) cellular therapy created ex vivo, aimed at treating individuals with solid tumors that exhibit overexpression of human epidermal growth factor receptor 2 (HER2).
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"The initiation of dosing for the first patient in the CT-0525 Phase 1 trial represents a major milestone in the advancement of engineered myeloid cells, as it marks the inaugural human testing of a CAR-Monocyte in the context of solid tumors," announced Eugene P. Kennedy, M.D., Chief Medical Officer at Carisma. "Our pre-clinical findings suggest that this innovative approach utilizing our CAR-M platform may surpass our initial CAR-Macrophage program in efficacy, particularly due to enhanced manufacturing speed, higher dosing capabilities, and improved potency, persistence, and tumor infiltration. We are eager to move forward with this trial and anticipate sharing preliminary results by the end of 2024."
"Patients with HER2-overexpressing solid tumors face an urgent demand for novel therapeutic strategies, as disease progression remains a frequent challenge," stated Davendra Sohal, M.D., M.P.H., Professor of Medicine at the University of Cincinnati Cancer Center. "CT-0525 offers a unique approach that could address this need, providing hope for patients with HER2-positive cancers. We are honored to be the first site treating a patient with CT-0525 and look forward to continued collaboration with Carisma and other cancer centers to expedite patient enrollment in the Phase 1 trial," Davendra Sohal added.
The Phase 1 clinical trial of CT-0525 is an open-label study aimed at evaluating the safety, tolerability, and manufacturing feasibility of CT-0525. The trial will include participants with locally advanced or metastatic solid tumors that overexpress HER2 and have shown progression following standard approved treatments. The study will feature two dose escalation cohorts.
Carisma will present a "Trial in Progress" poster detailing the design of the CT-0525 Phase 1 trial at the American Society of Clinical Oncology 2024 Annual Meeting, which will take place from May 31 to June 4, 2024, in Chicago, IL.
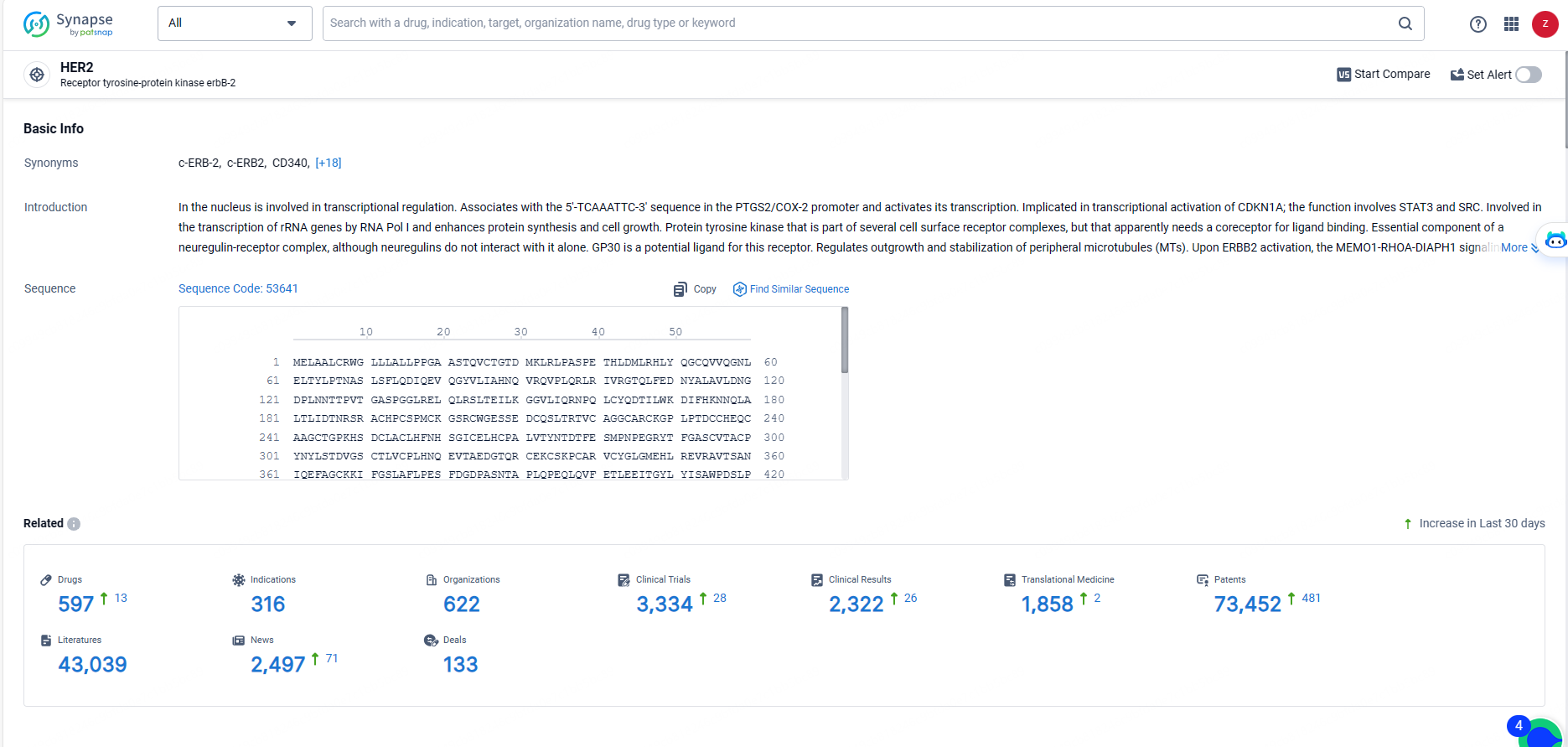
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 20, 2024, there are 597 investigational drugs for the HER2 targets, including 316 indications, 622R&D institutions involved, with related clinical trials reaching 3334, and as many as 73452 patents.
As an expert in the pharmaceutical industry, it is important to closely monitor the progress of CT-0525 as it advances through the clinical development process. The successful development of a CAR-M therapy targeting HER2 positive solid tumors could represent a significant advancement in the treatment of certain types of cancer, potentially offering new hope for patients with limited treatment options. Additionally, understanding the competitive landscape and potential partnerships or collaborations in the field of CAR-M therapies for neoplasms could provide valuable insights for business development opportunities in the pharmaceutical industry.