Celldex Reports Successful Phase 2 Results for Barzolvolimab in Chronic Inducible Urticaria
Celldex Therapeutics, Inc. unveiled favorable top-line outcomes from the Phase 2 clinical study of barzolvolimab in chronic inducible urticaria (CIndU), specifically focusing on cold urticaria and symptomatic dermographism. The trial involved individuals experiencing symptoms even after receiving antihistamine therapy.
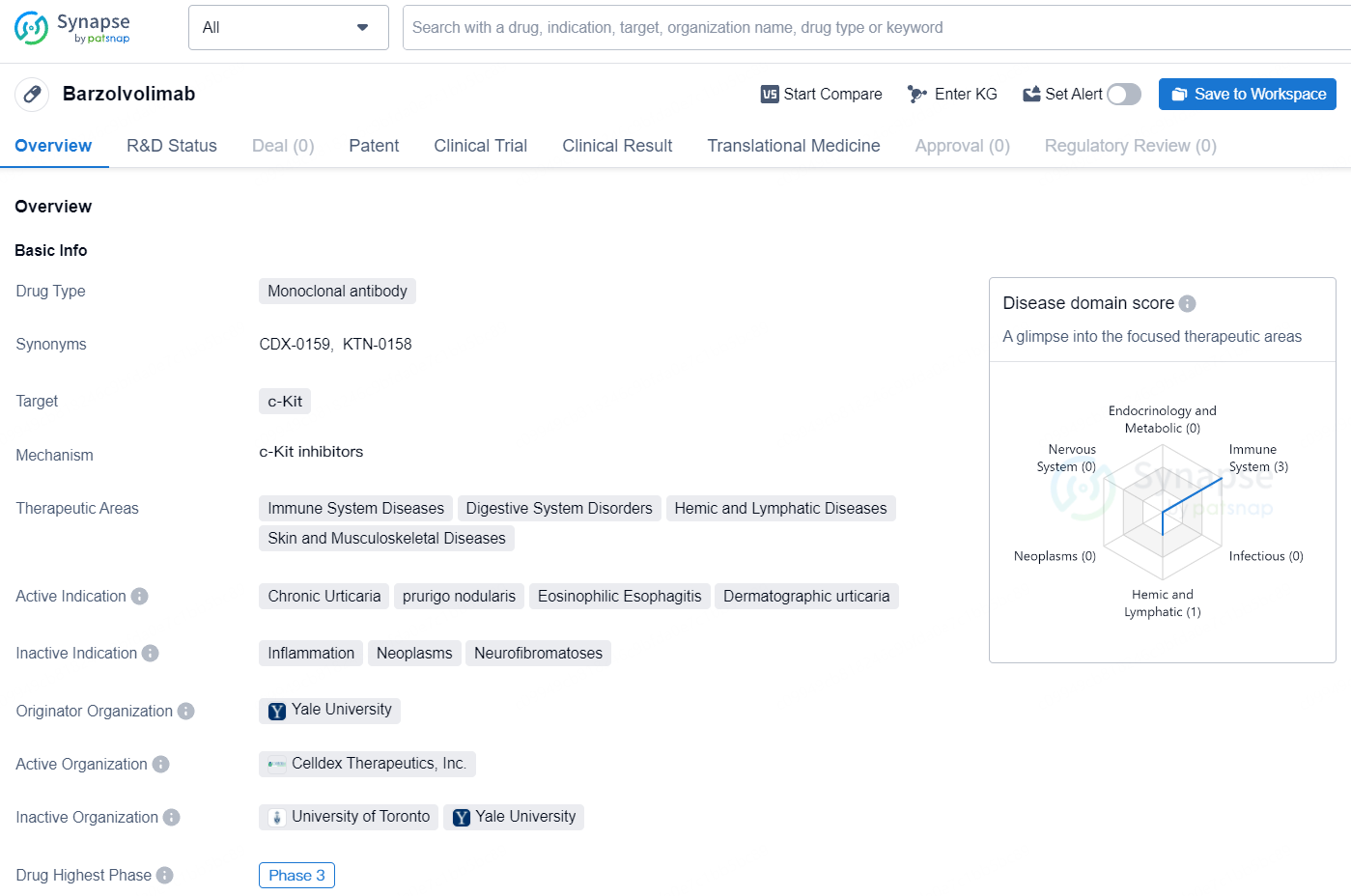
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Barzolvolimab is a humanized monoclonal antibody that specifically targets the receptor tyrosine kinase KIT, effectively blocking its essential function for mast cell survival and activity. Chronic Inducible Urticaria (CIndU) is characterized by the presence of hives triggered by specific factors like exposure to cold temperatures in ColdU and skin irritation in SD, with mast cell activation being a key factor in these conditions.
"Barzolvolimab has shown promising results in a significant placebo-controlled study for chronic inducible urticaria, offering hope for patients," stated Anthony S. Marucci, President and CEO of Celldex Therapeutics.
Inducible urticaria poses significant challenges for patients, impacting their lives with severe itching and hives. The progression of Barzolvolimab into registration studies aims to provide a new treatment option for patients suffering from this condition.
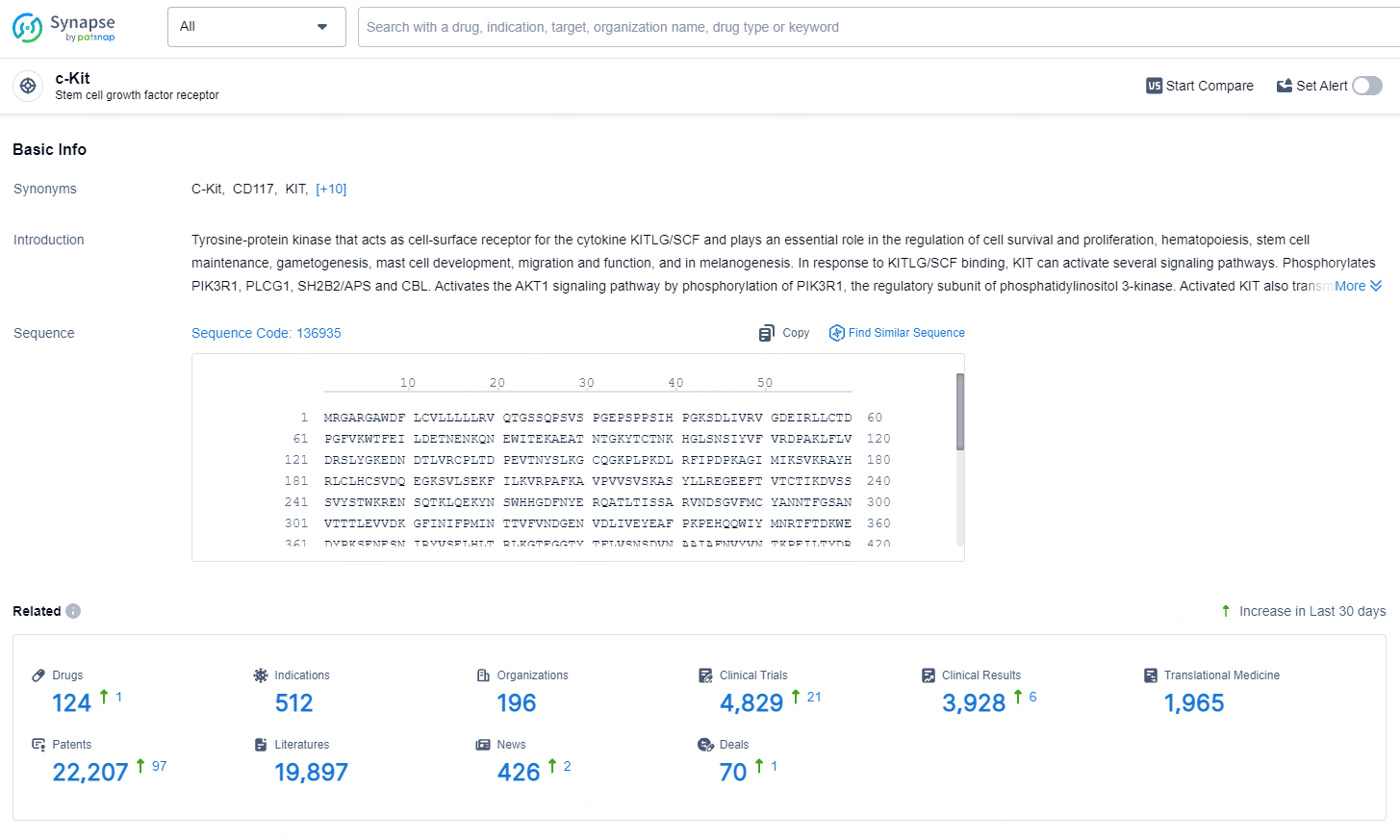
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 2, 2024, there are 124 investigational drugs for the c-Kit targets, including 512 indications, 196 R&D institutions involved, with related clinical trials reaching 4829, and as many as 22207 patents.
Barzolvolimab as a monoclonal antibody targeting c-Kit represents a potential advancement in the treatment of chronic urticaria, prurigo nodularis, eosinophilic esophagitis, and dermatographic urticaria. As it progresses through clinical trials, additional data will be needed to assess its safety and effectiveness for these indications.