undefined European Commission gives green light to Roche's Vabysmo for treating retinal vein occlusion (RVO)
Roche disclosed today that the European Commission has given the green light to Vabysmo® (faricimab) for managing visual impairment caused by macular edema linked to retinal vein occlusion. RVO marks the third condition approved for Vabysmo in the European Union, alongside neovascular or ‘wet’ age-related macular degeneration and diabetic macular edema. These three retinal disorders collectively impact nearly 80 million individuals globally and rank as key contributors to vision impairment.
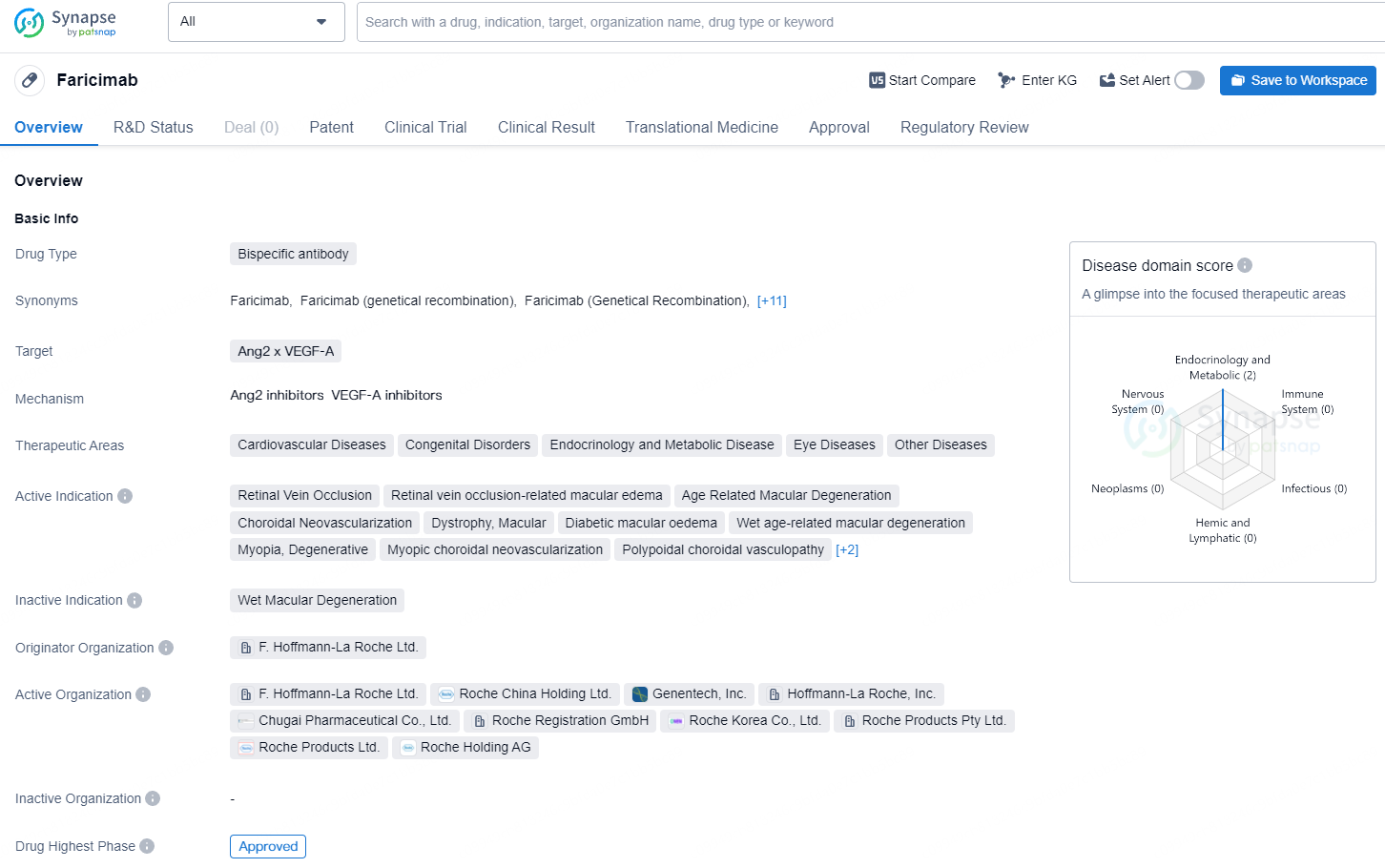
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Roche's Chief Medical Officer and Head of Global Product Development, Levi Garraway, M.D., Ph.D., stated, "Vabysmo offers a new treatment choice for European individuals with retinal vein occlusion that can help maintain and enhance vision, while also promoting retinal drying. The effectiveness and safety of Vabysmo have been proven in worldwide clinical trials, with a growing body of real-world evidence supporting it, as thousands of individuals around the globe have been treated."
The approval is founded on positive outcomes from two international Phase III BALATON and COMINO trials, which assessed Vabysmo in over 1,200 individuals with macular edema due to branch and central retinal vein occlusion.
Prof. Frank Holz, chair and professor of the Department of Ophthalmology at the University of Bonn, Germany, remarked, "Individuals with retinal vein occlusion have limited therapy choices that necessitate frequent clinic visits. This endorsement could have a substantial impact on those with retinal vein occlusion and their caretakers, who are faced with managing the debilitating effects of their condition on daily activities such as driving, reading, socializing, traveling, and pursuing hobbies."
The findings showed that monthly Vabysmo treatment resulted in early and sustained vision improvement in individuals with BRVO and CRVO, meeting the primary goal of comparable gains in visual acuity at 24 weeks compared to aflibercept. Moreover, data indicated that Vabysmo led to swift and strong drying of retinal fluid. Retinal drying is crucial in clinical evaluation, as ocular swelling due to excessive fluid in the eye has been associated with distorted and blurry vision.
Vabysmo is the initial and sole bispecific antibody authorized for ocular use, uniquely designed to target and block two signaling pathways that are associated with various vision-threatening retinal conditions, through the inhibition of angiopoietin-2 (Ang-2) and vascular endothelial growth factor-A (VEGF-A) to reinstate vascular stability.
Originally approved for RVO by the United States Food and Drug Administration in October 2023 and by the Japan Ministry of Health, Labour and Welfare in March 2024, Vabysmo is currently accessible in nearly 100 countries for individuals with nAMD and DME, with over four million doses distributed worldwide.
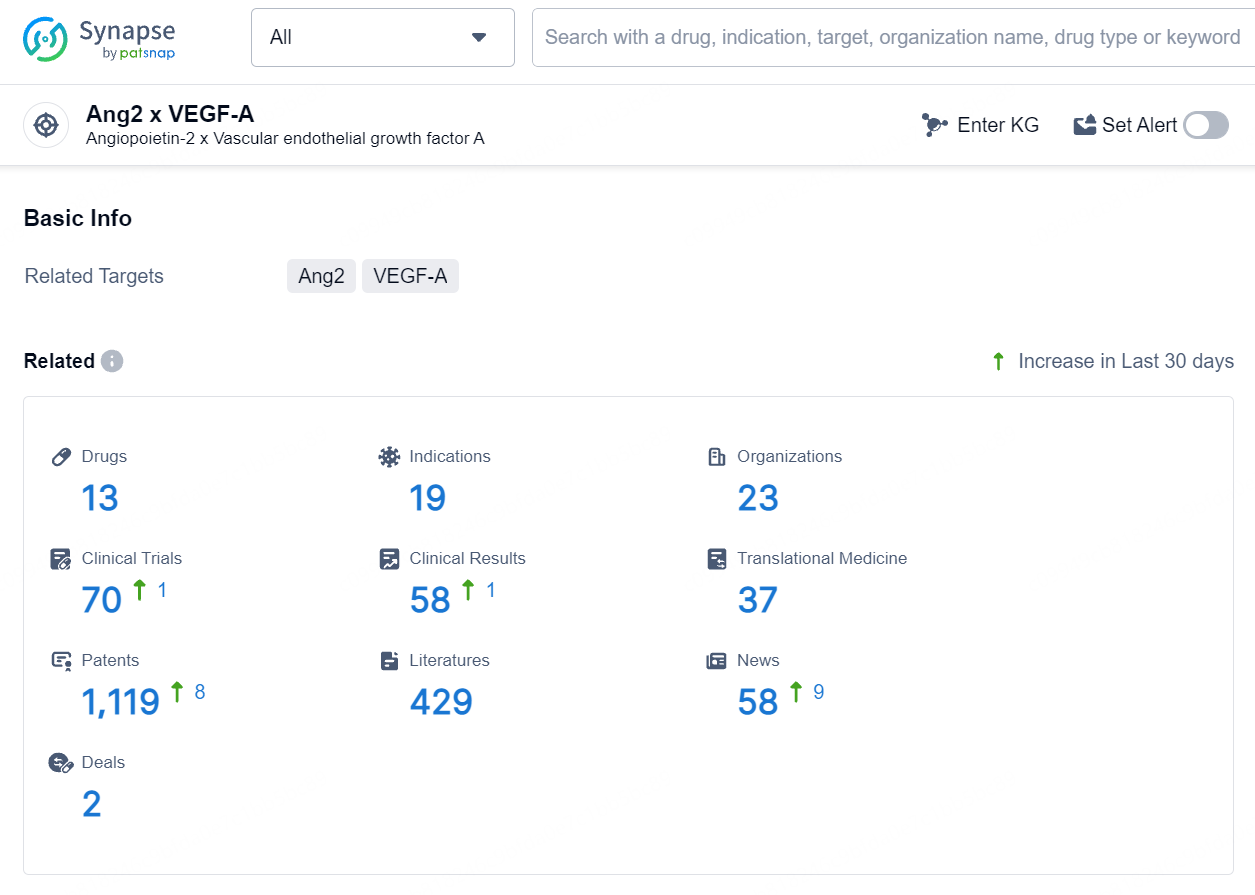
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of August 2, 2024, there are 13 investigational drugs for the Ang2 and VEGF-A target, including 19 indications, 23 R&D institutions involved, with related clinical trials reaching 70, and as many as 1119 patents.
The unique bispecific nature of faricimab, targeting both Ang2 and VEGF-A, makes it a promising treatment option for a wide range of eye diseases and related conditions. The approval of faricimab represents a significant advancement in the treatment of these conditions, offering new options for patients and healthcare providers. The drug's approval in both the United States and China further underscores its potential to address unmet medical needs in these regions.