Crestone Releases Encouraging Phase 2 Trial Results for CRS3123 in Treating C. Difficile Infections (CDI)
Crestone, Inc. ("Crestone") announced promising initial results from its Phase 2 clinical trial of CRS3123 in patients with Clostridioides difficile infection (CDI). CRS3123, a novel therapeutic agent, demonstrated limited spectrum activity and negligible effects on normal gut microbiota in preclinical and Phase 1 studies. The primary intent-to-treat (ITT) analysis encompassed 43 patients, showing consistent clinical cure rates at the day 12 test-of-cure visit across all three treatment groups. Specifically, 28 out of 29 patients (97%) administered either of the two dosages of CRS3123 achieved clinical cure, in comparison to 13 out of 14 patients (93%) receiving vancomycin. No instances of clinical failure were noted at the day 12 evaluation, although results for two patients remained inconclusive.
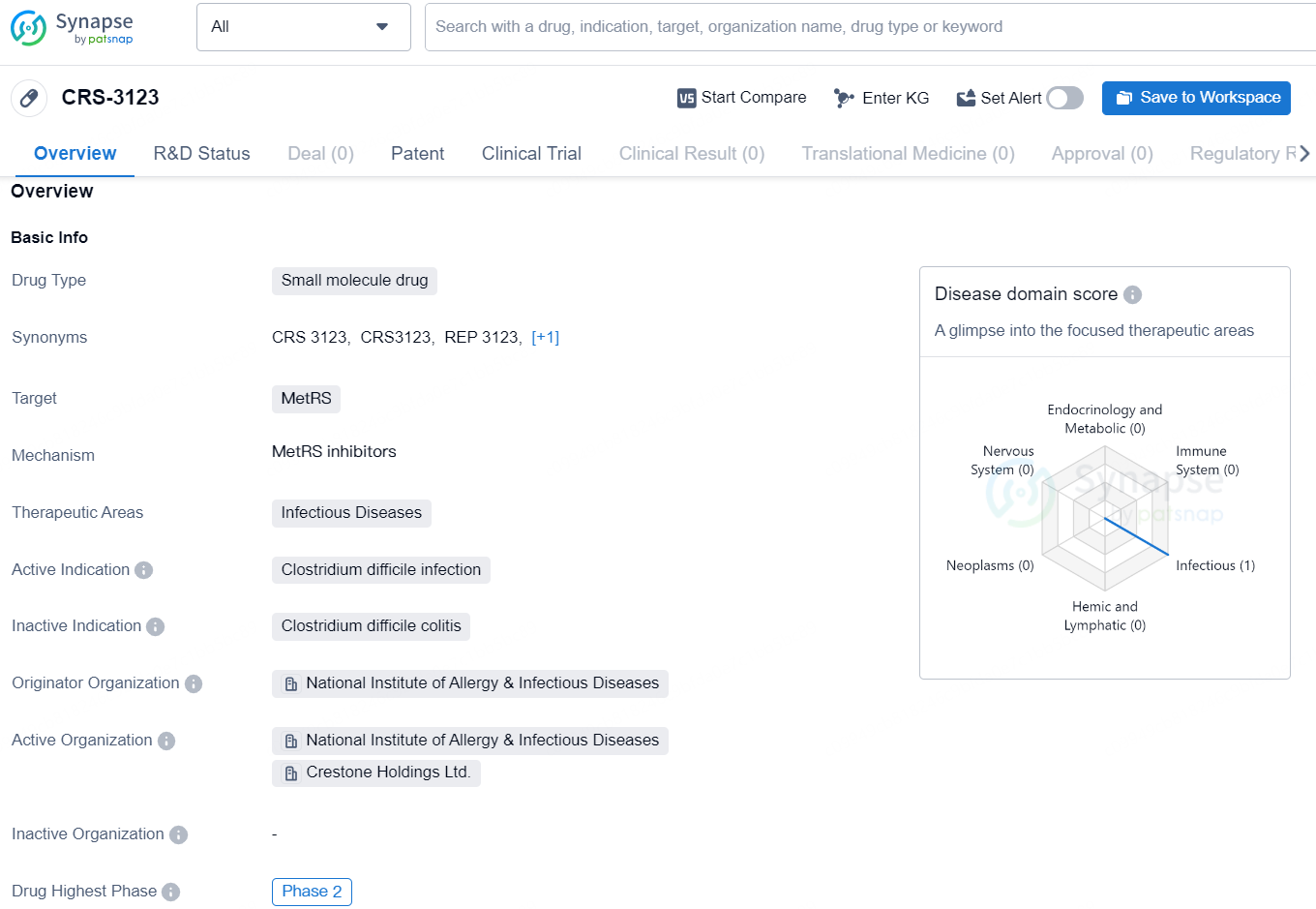
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Noticeably, patients receiving CRS3123 experienced a substantial drop in CDI recurrence rates. For example, by the 40th day, the recurrence rate was 4% for CRS3123 compared to 23% for vancomycin. CRS3123's tolerability was high, with no significant adverse events linked to the treatment. Crestone plans to showcase the Phase 2 trial outcomes at a forthcoming medical conference.
CDI remains the most prevalent hospital-acquired infection in the United States and has increasingly spread within the community, even impacting younger demographics. The U.S. records almost half a million cases per year, with approximately 30,000 related fatalities.
Dr. Thomas Louie of the University of Calgary in Canada, who led the research, stated: “Tackling C. difficile infection urgently requires therapies that maintain gut microbiota, aiding in microbiome recovery and preventing recurrent CDI. The results from this study suggest that CRS3123 is a favorable candidate for further investigation.”
The study’s epidemiological analysis and toxin assays were performed at the University of Leeds. Dr. Mark Wilcox, a professor at Leeds Teaching Hospitals and the University of Leeds, as well as the CDI Lead for the UK Health Security Agency, noted: “Effectively addressing C. difficile while sustaining healthy gut flora is the aim of CDI therapies. This Phase 2 study’s findings support that goal.”
Dr. Jon Bruss, Interim Chief Medical Officer at Crestone, commented: “We are thrilled with these results. Conducting this study was tough due to the pandemic’s strain on healthcare systems. We profoundly appreciate the contributions of the patients, their families, the clinicians, and our partners to these essential data.”
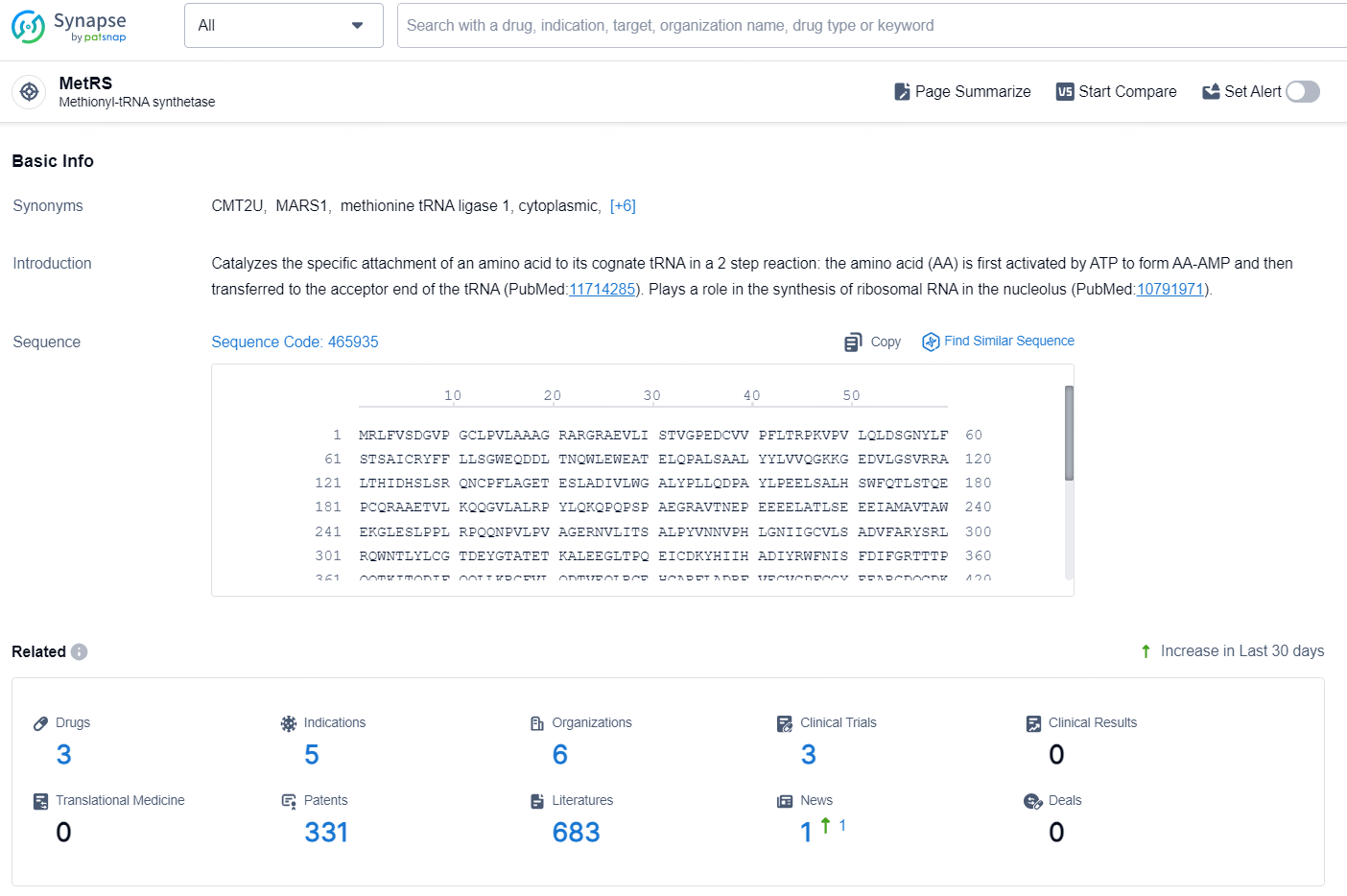
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 9, 2024, there are 3 investigational drug for the MetRS targets, including 5 indications, 6 R&D institutions involved, with related clinical trials reaching 3, and as many as 331 patents.
CRS-3123 is a small molecule drug that targets MetRS and falls under the therapeutic area of Infectious Diseases, specifically active against Clostridium difficile infection. The originator organization of this drug is the National Institute of Allergy & Infectious Diseases. As of now, CRS-3123 has reached the highest phase of development globally, which is Phase 2.