Datopotamab Deruxtecan: A Novel ADC Targeting Trop-2 for Advanced Breast Cancer
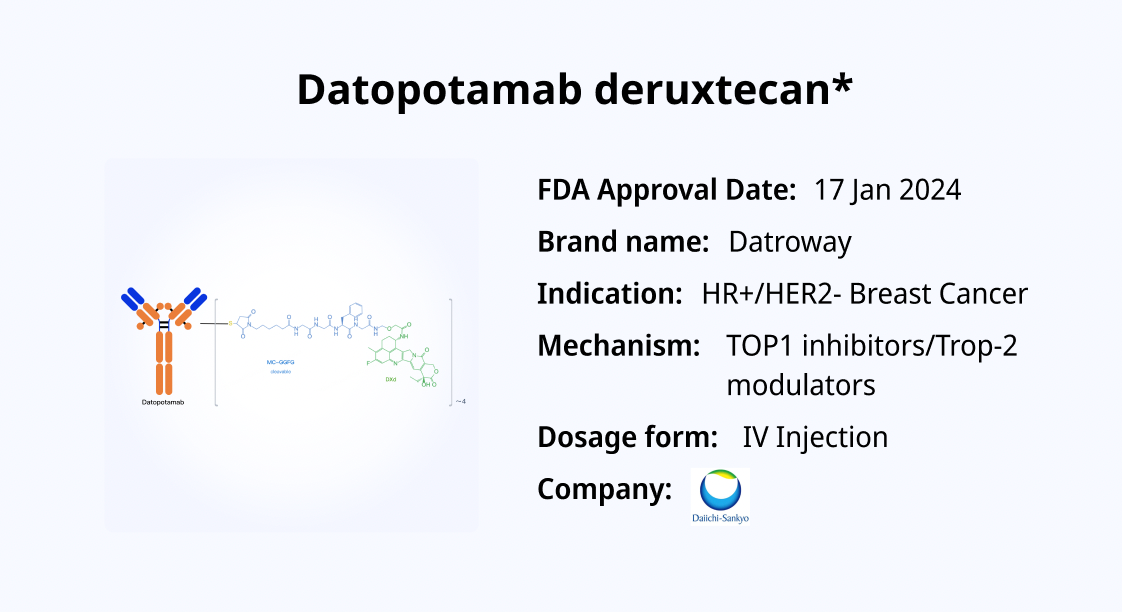
Datopotamab Deruxtecan (Dato-DXd) is a Trop-2-targeting antibody-drug conjugate (ADC) developed jointly by Daiichi Sankyo and AstraZeneca. On December 27, 2024, datopotamab deruxtecan received approval in Japan for the treatment of adult patients with unresectable or metastatic HR-positive/HER2-negative breast cancer who have previously received chemotherapy. Subsequently, on January 17, 2025, the U.S. Food and Drug Administration (FDA) also approved datopotamab deruxtecan for the treatment of adult patients with unresectable or metastatic HR-positive, HER2-negative breast cancer who have undergone prior systemic therapy.
Mechanism of Action
Datopotamab deruxtecan is an ADC that combines a tumor-specific monoclonal antibody with a potent cytotoxic payload, enabling targeted delivery of the cytotoxic agent to tumor cells. The core component of datopotamab deruxtecan is a humanized monoclonal antibody targeting Trop-2. Trop-2 (also known as tumor-associated calcium signal transducer 2, TACSTD2) is a transmembrane glycoprotein highly expressed in various human cancers, including but not limited to breast cancer, lung cancer, and gastrointestinal cancers.
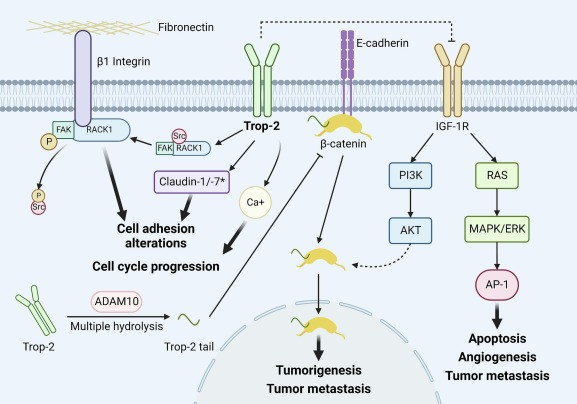
Trop-2 is not merely a tumor marker but also plays a crucial role in cancer progression. Studies have shown that Trop-2 is involved in cell proliferation, migration, and invasion and is associated with poor prognosis in several cancers. Additionally, Trop-2 contributes to tumor microenvironment regulation and angiogenesis, making it an attractive therapeutic target.
Datopotamab deruxtecan’s monoclonal antibody specifically recognizes and binds to Trop-2 on the surface of cancer cells. This targeted binding minimizes off-target effects, thereby reducing the risk of adverse reactions. Once bound to Trop-2, the entire ADC complex undergoes endocytosis, entering the cancer cell.
In addition to its targeting mechanism, datopotamab deruxtecan carries a highly potent cytotoxic payload—deruxtecan (DXd), a topoisomerase I inhibitor. DXd is released within the cancer cell, interfering with DNA replication and ultimately leading to cancer cell death. The linker is designed to remain stable in circulation and release DXd only under specific intracellular conditions, preventing premature release and limiting toxicity to normal tissues.
Key Drug Characteristics
Datopotamab deruxtecan utilizes an enzymatically cleavable tetrapeptide linker (GGFG) along with a self-immolative moiety and a maleimide-based conjugation strategy. This design ensures stability in circulation while enabling targeted release of DXd upon lysosomal degradation inside the cancer cell. Additionally, due to its unique structural properties, DXd exhibits a “bystander effect,” meaning that even neighboring tumor cells that do not directly express Trop-2 can be affected by DXd diffusion, enhancing its therapeutic efficacy.
Upon binding to Trop-2-positive tumor cells, the ADC complex is internalized. The acidic intracellular environment triggers linker cleavage, releasing DXd. DXd then diffuses into the nucleus, where it disrupts DNA replication, halting cancer cell proliferation and inducing apoptosis. Despite its potent cytotoxic activity, datopotamab deruxtecan’s targeted delivery mechanism significantly reduces toxicity to non-cancerous tissues, thereby improving treatment safety. Clinical trials have demonstrated that the incidence of ≥ Grade 3 treatment-related adverse events is lower than that of standard therapies, indicating favorable tolerability.
Datopotamab deruxtecan’s therapeutic efficacy is driven by its highly specific Trop-2 targeting, its potent cytotoxic payload DXd, and its advanced linker technology, which ensures stability and controlled drug release in vivo. This mechanism not only enhances the drug’s antitumor activity but also improves patient quality of life and treatment safety.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!
Refrence
- 1. Qiu S, Zhang J, Wang Z, Lan H, Hou J, Zhang N, Wang X, Lu H. Targeting Trop-2 in cancer: Recent research progress and clinical application. Biochim Biophys Acta Rev Cancer. 2023 Jul;1878(4):188902. doi: 10.1016/j.bbcan.2023.188902. Epub 2023 Apr 29. PMID: 37121444.