Fresenius Boosts Its Pharma Performance and Introduces Tyenne®, the Third Biosimilar to Receive U.S. Approval
Fresenius has declared through its subsidiary, Fresenius Kabi, the launch of Tyenne (tocilizumab-aazg) in the United States. This product is a biosimilar to Actemra (tocilizumab) and is now readily available. Tyenne is designed for the management of chronic autoimmune conditions and is distributed as an intravenous formulation.
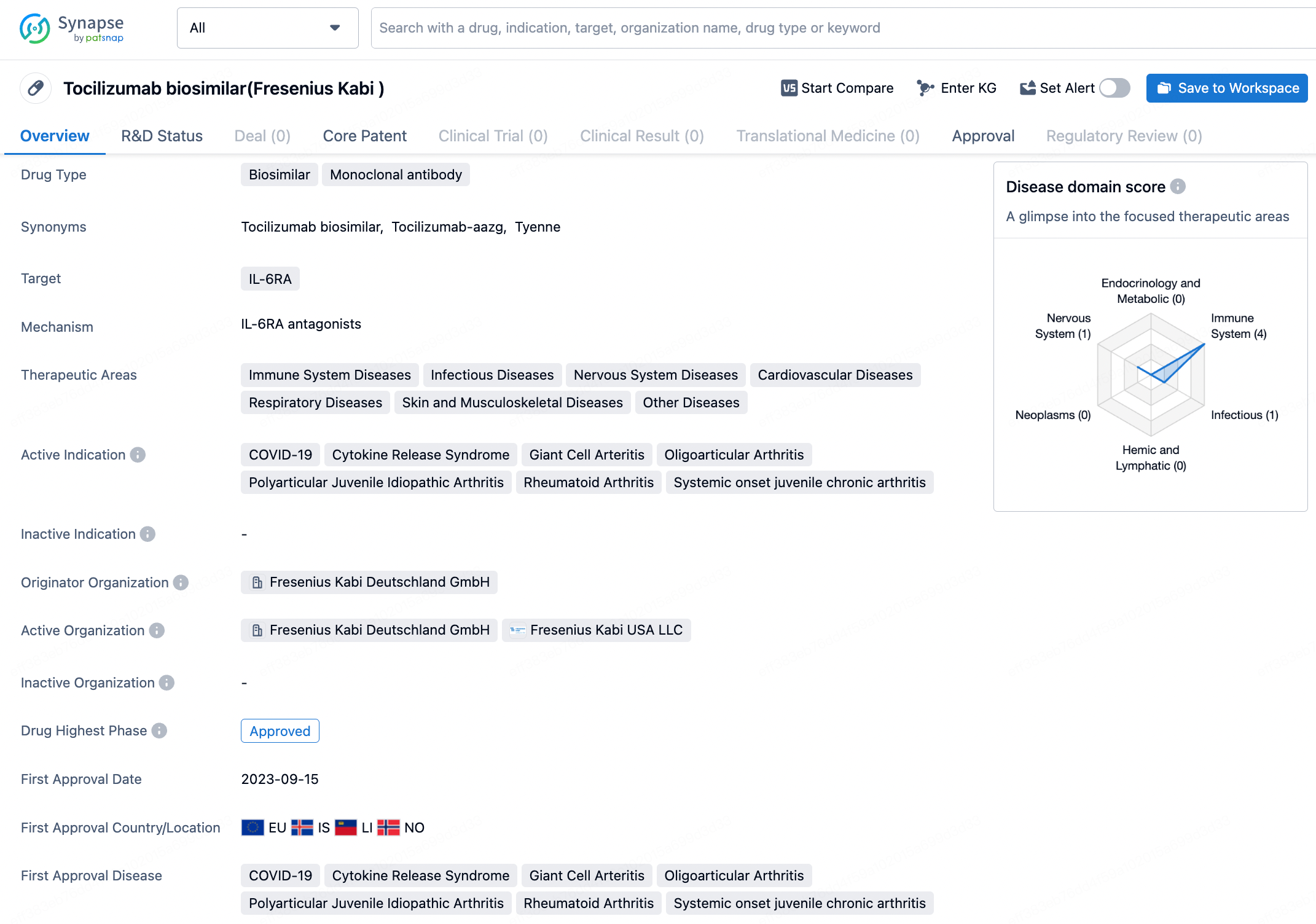
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Michael Sen, CEO of Fresenius, stated: “The introduction of Tyenne in the U.S. marks a significant step forward in boosting our robust Pharma sector. Expanding this sector is a key part of our #FutureFresenius initiative. So far in 2024, our Pharma division has performed exceptionally well. We are especially pleased with the developments at our majority-held biotech firm mAbxience and the market reception of Tyenne, Europe’s initial tocilizumab biosimilar, released in November 2023.”
Tyenne is distinctive as the first tocilizumab biosimilar to offer both intravenous and subcutaneous administration, receiving Food and Drug Administration (FDA) endorsement on March 5, 2024. It is the third biosimilar by Fresenius approved in the U.S. and the second to be part of their immunology line. This biologic medication is designed to treat various autoimmune ailments including rheumatoid arthritis, giant cell arteritis, and both polyarticular and systemic juvenile idiopathic arthritis.
Pierluigi Antonelli, CEO of Fresenius Kabi, commented: “Tyenne will reshape how inflammatory and immune diseases are managed in the U.S. Expanding our reach to more patients with our cutting-edge biopharmaceutical products is setting a strong growth trajectory in a key market area. We’re poised to advance our extensive array of biosimilars for autoimmune diseases and cancer, with numerous candidates nearing the final stages of development.”
Fresenius Kabi backs health care professionals and patients through its comprehensive support program, which makes a variety of treatment avenues more accessible and enhances the sustainability of health care systems.
Last year, Fresenius took decisive steps to establish itself as a premier therapy-centered corporation. Aligned with this strategic vision, Fresenius has restructured for greater efficiency, focused its attention on key operating companies Fresenius Kabi and Fresenius Helios, and is committed to ongoing improvement in its operational performance.
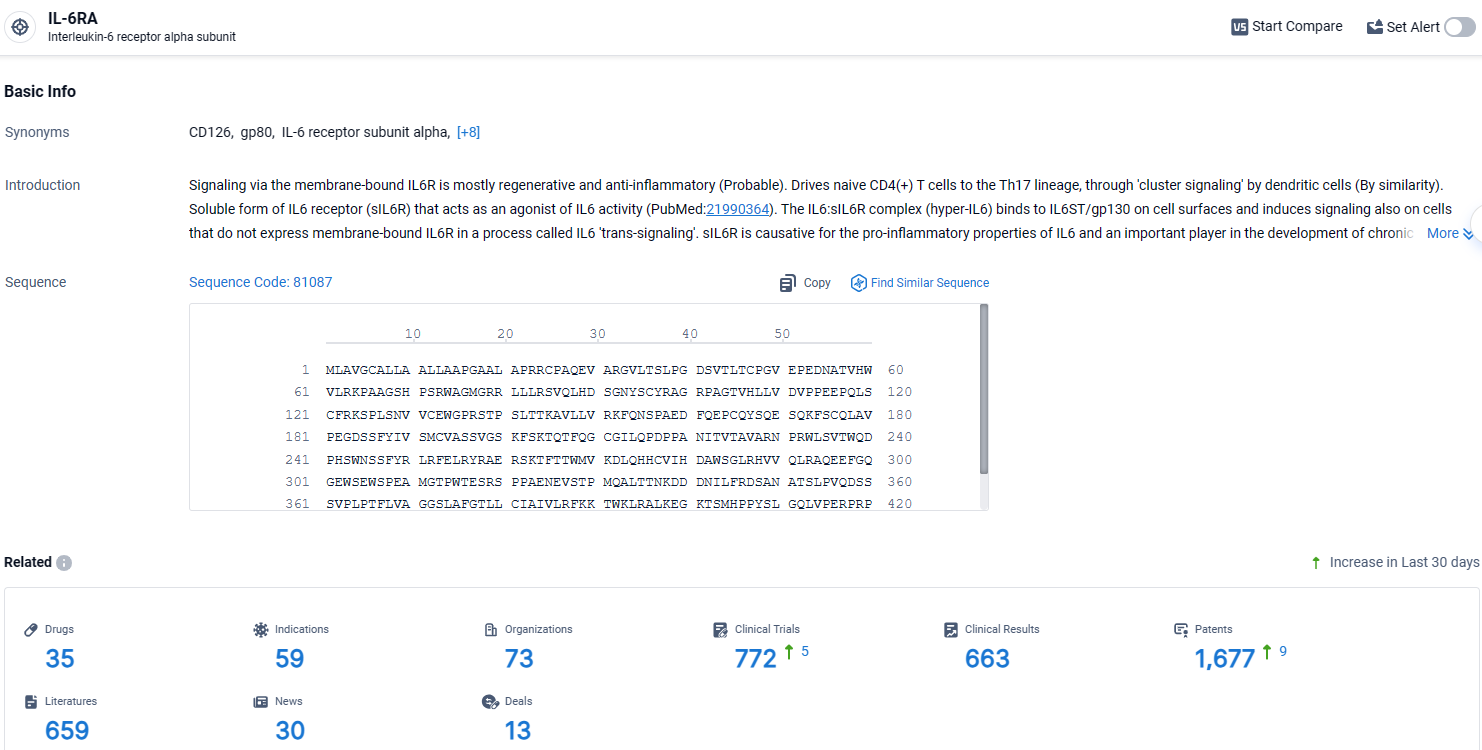
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of April 17, 2024, there are 35 investigational drugs for the IL-6RA target, including 59 indications, 73 R&D institutions involved, with related clinical trials reaching 772, and as many as 1677 patents.
Tyenne specifically targets IL-6RA, which is involved in various immune system diseases, infectious diseases, nervous system diseases, cardiovascular diseases, respiratory diseases, skin and musculoskeletal diseases, as well as other diseases. Tyenne shows promise as a valuable addition to the pharmaceutical market, offering a biosimilar alternative for the treatment of various immune system and inflammatory diseases. Its approval in multiple indications and its expected launch in the near future highlight the potential impact it may have on patient care and healthcare systems.