Exploring Cadonilimab's R&D successes and its clinical results at the 2024 AACR
PD-1 inhibitors plus chemo have shown efficacy in first-line treatment of advanced G/GEJ adenocarcinoma; however, the survival benefits are limited in patients with low PD-L1 expression. On April 7, 2024, the latest clinical data of Cadonilimab (the PD-1/CTLA-4 bispecific antibody) plus chemo were reported in 2024 AACR.
Cadonilimab's R&D Progress
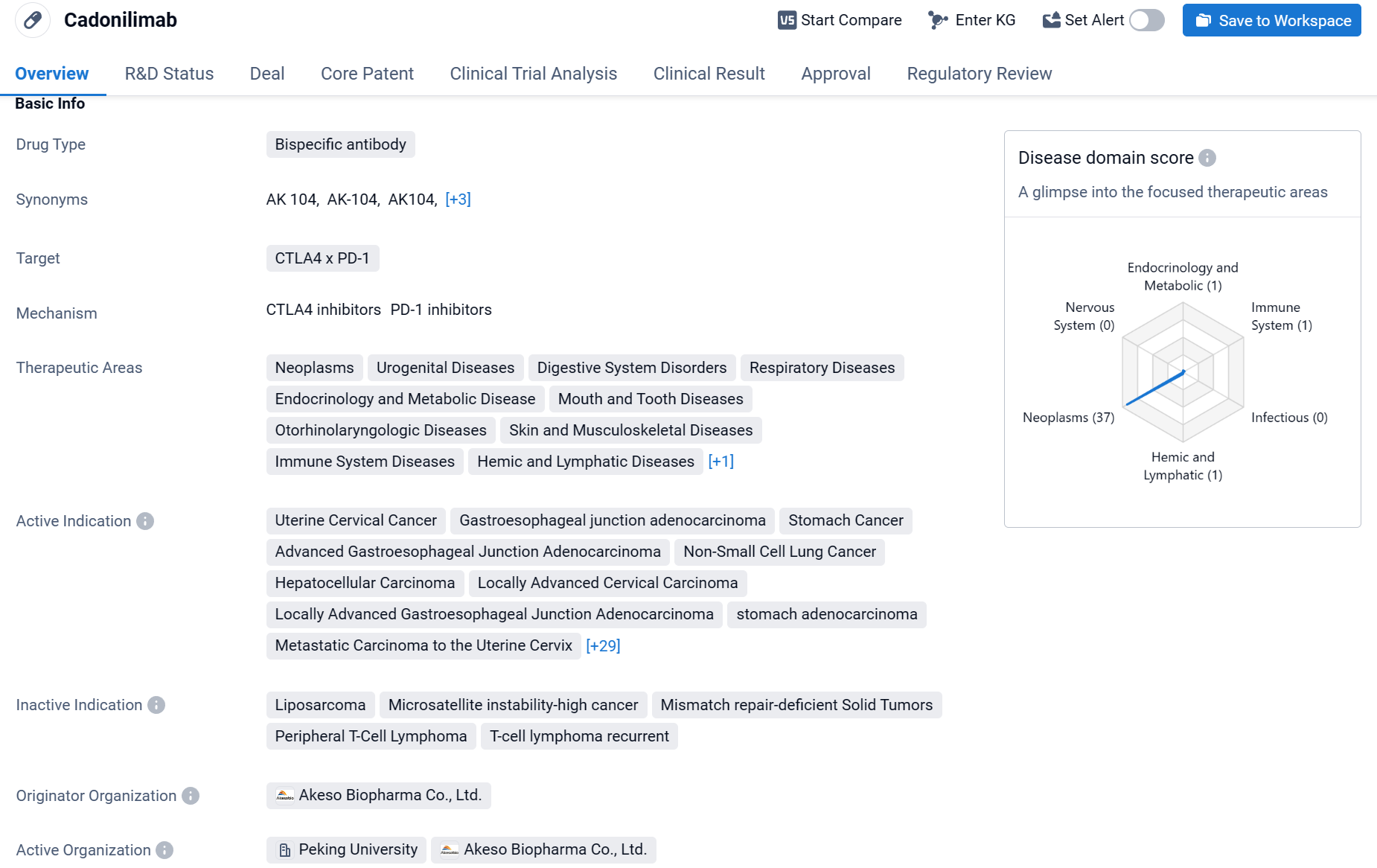
Cadonilimab is a bispecific antibody drug that targets CTLA4 x PD-1. It has been approved for use in various therapeutic areas, including neoplasms, urogenital diseases, digestive system disorders, respiratory diseases, endocrinology and metabolic disease, mouth and tooth diseases, otorhinolaryngologic diseases, skin and musculoskeletal diseases, immune system diseases, hemic and lymphatic diseases, and other diseases.
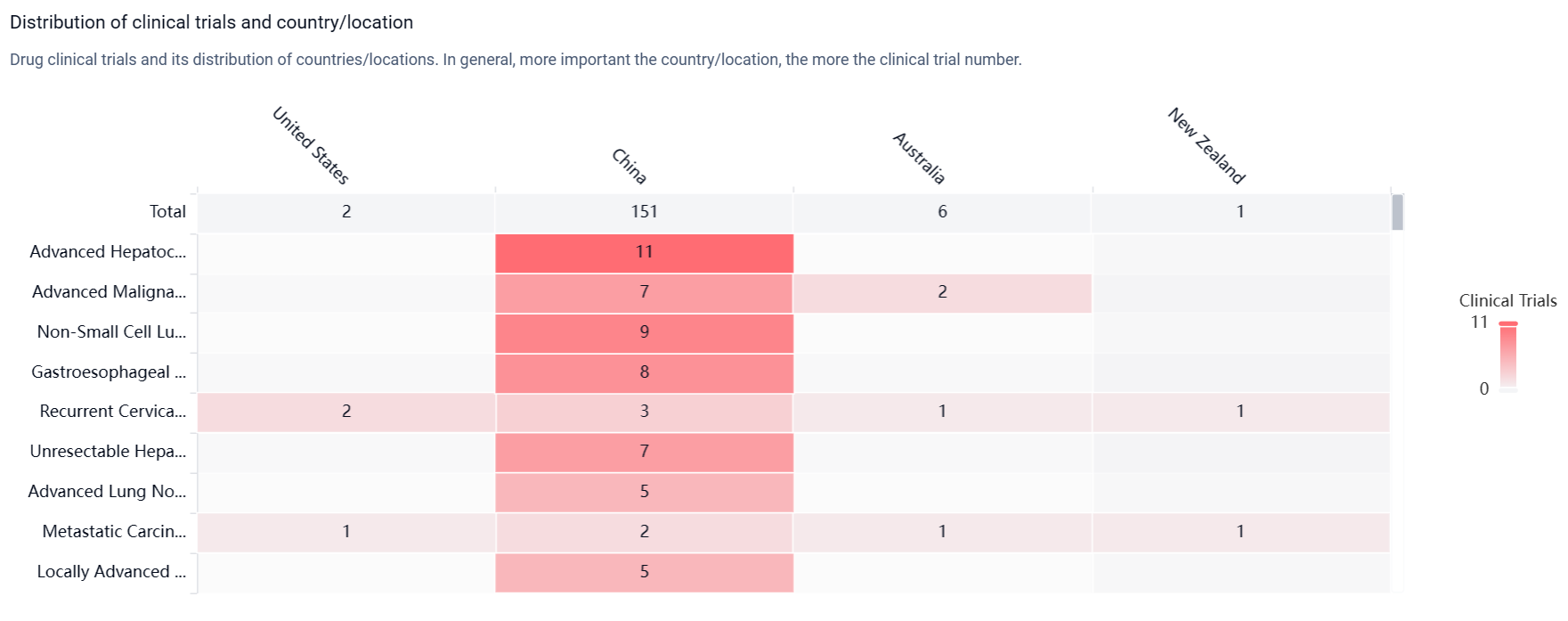
According to the Patsnap Synapse, Cadonilimab has received approval in the highest phase globally and in China. And the clinical trial distributions for Cadonilimab are primarily in the United States, China and Australia. The key indication is Advanced Hepatocellular Carcinoma.
Detailed Clinical Result of Cadonilimab
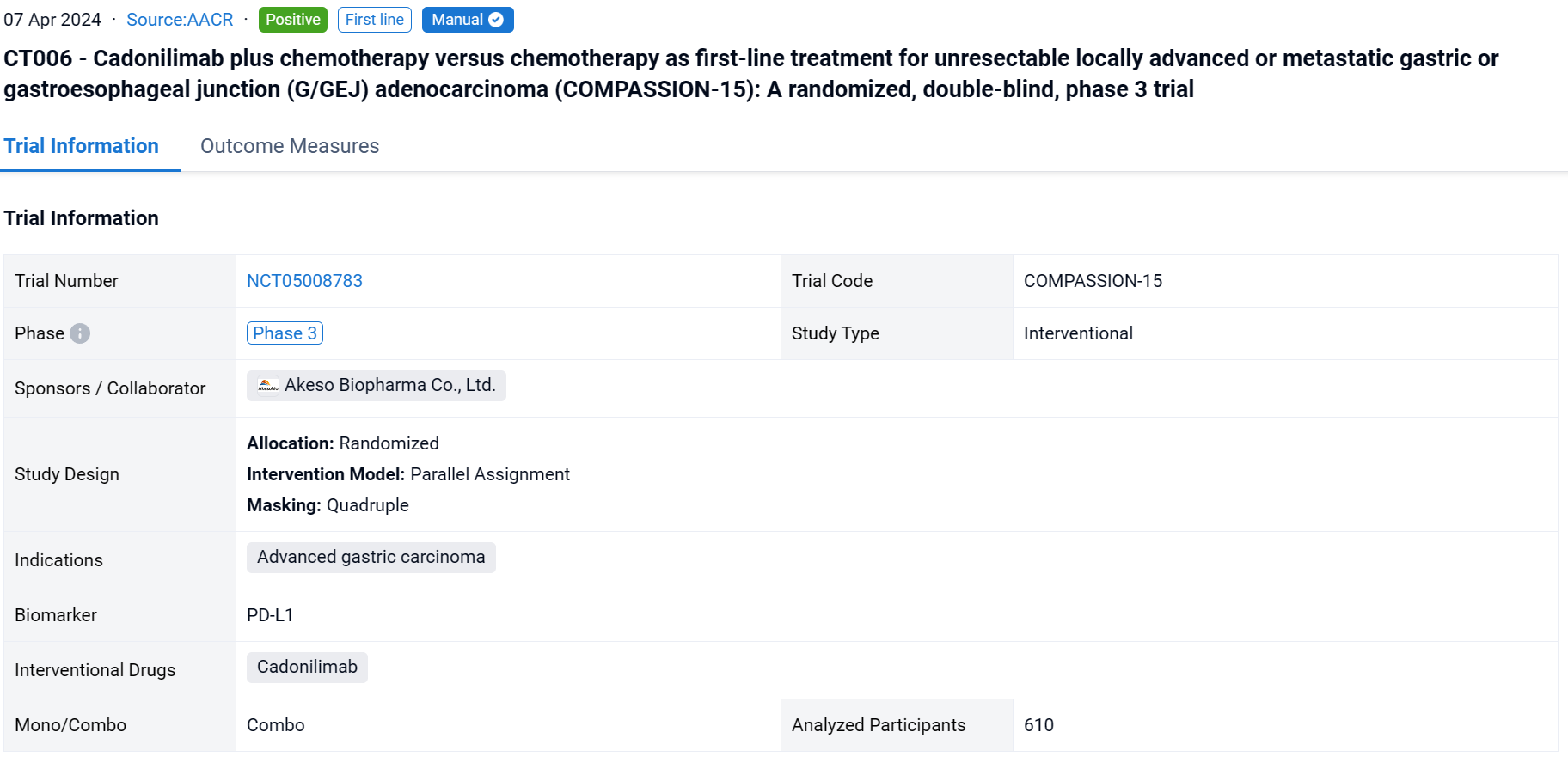
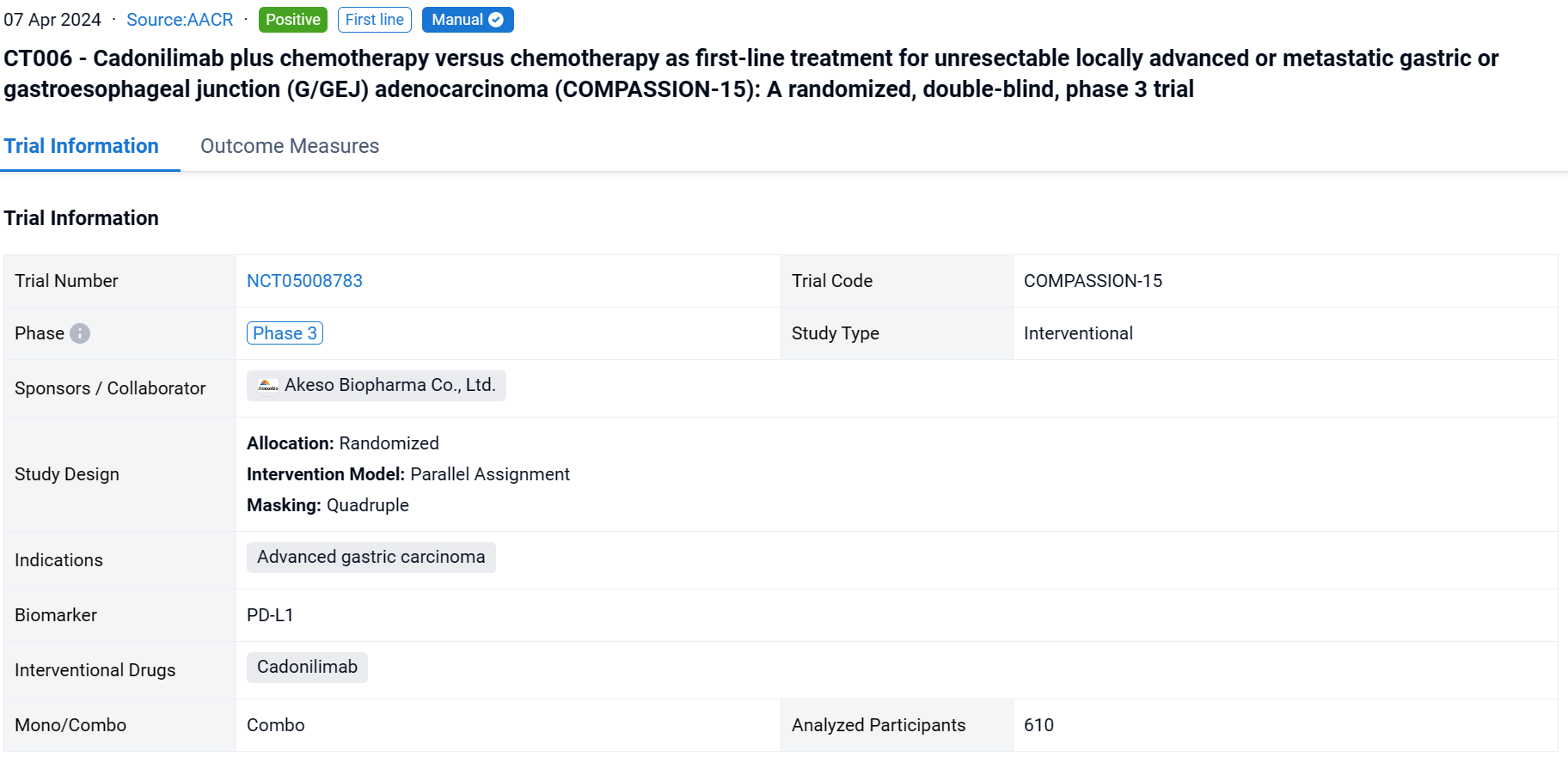
This randomized, double-blind, placebo-controlled phase 3 study (NCT05008783) was aimed to evaluate the efficacy and safety of cadonilimab, the PD-1/CTLA-4 bispecific antibody, plus chemo vs chemo alone as the first-line treatment in G/GEJ adenocarcinoma.
In this study, eligible pts were adults with untreated, unresectable, locally advanced, or metastatic G/GEJ adenocarcinoma, regardless of PD-L1 expression. Pts were randomized 1:1 to receive cadonilimab (10mg/kg Q3W) plus chemo (Q3W) or placebo plus chemo. Randomization was stratified by ECOG PS score (0 vs. 1), PD-L1 status (visualized combined positive score by Ventana SP263, vCPS<5% vs ≥5%), and hepatic metastasis (yes vs no). The primary endpoint was OS in the ITT population. The cutoff date was Aug 18, 2023.
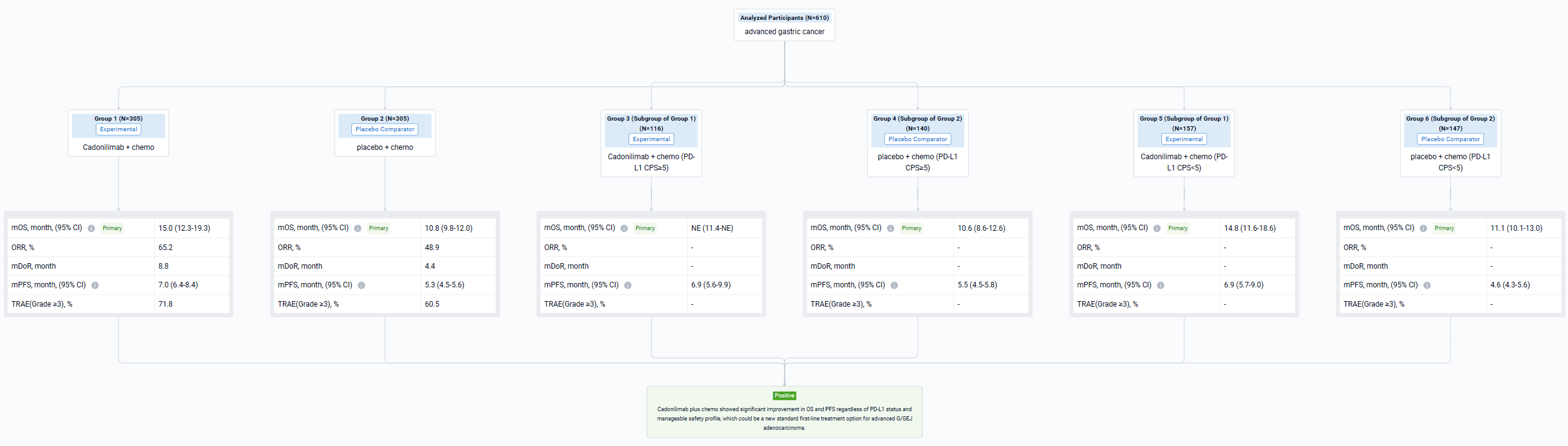
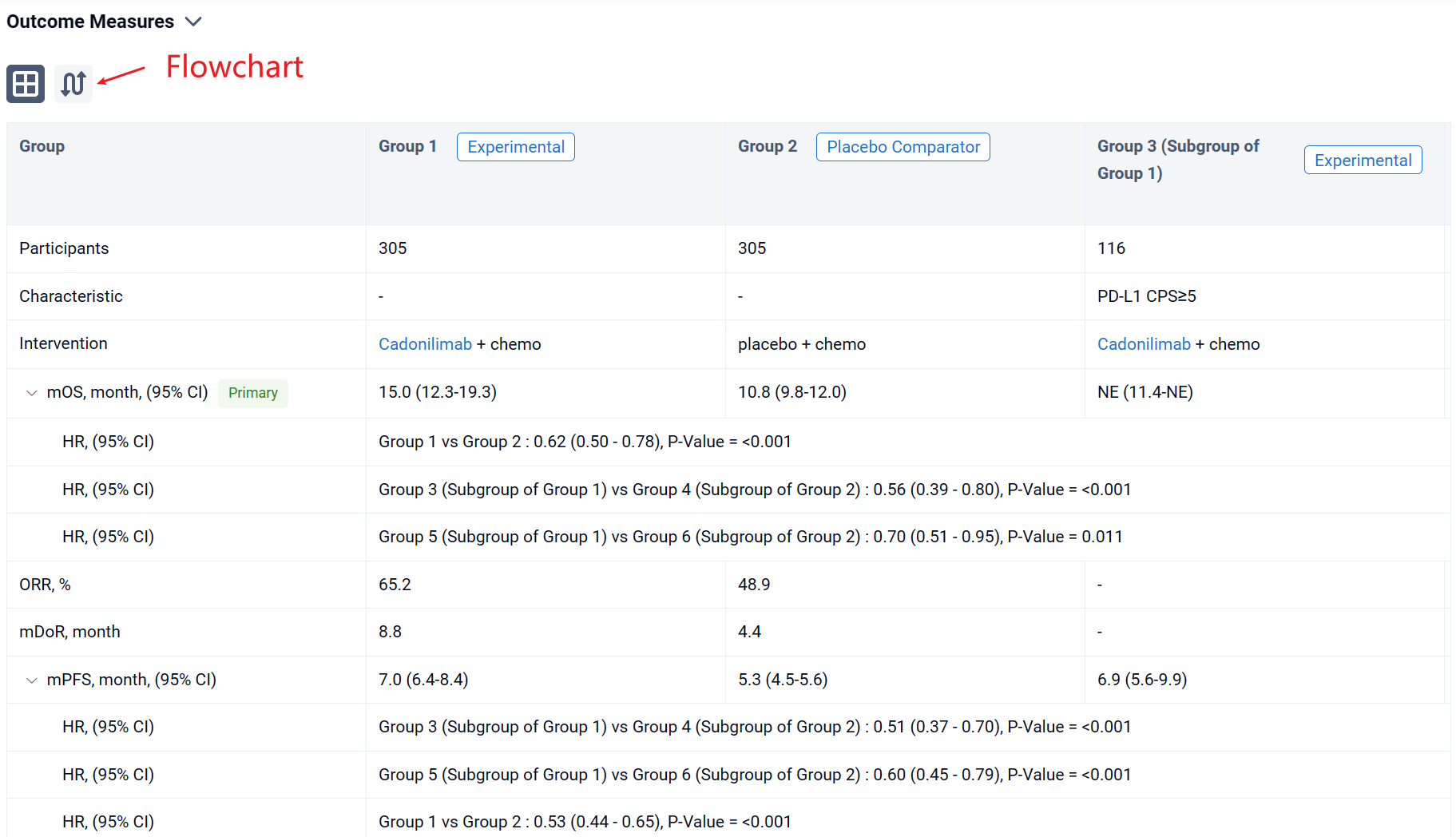
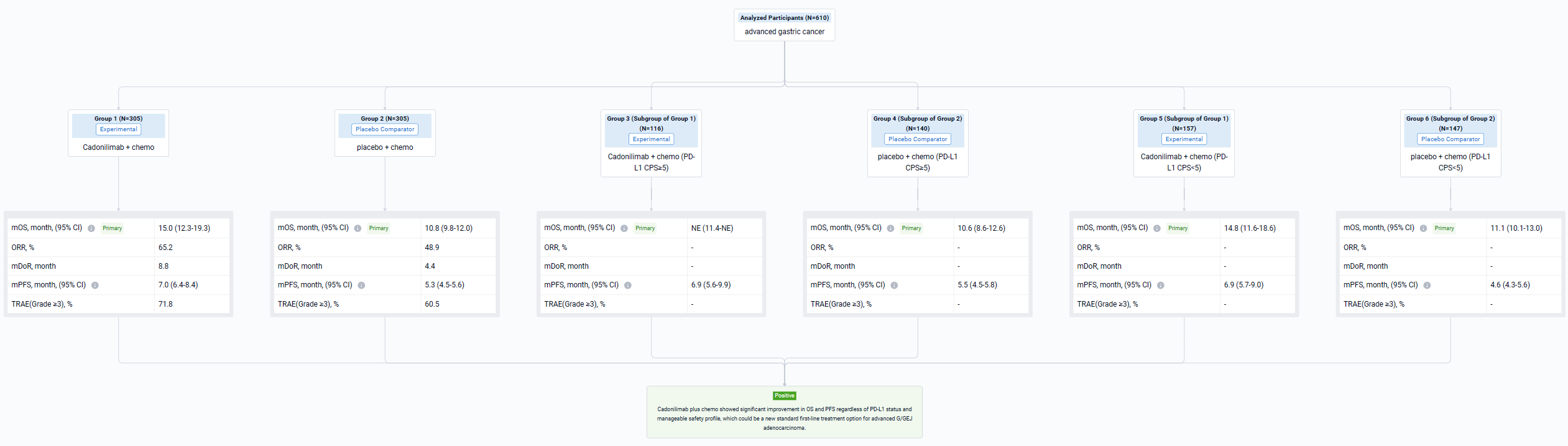
The result showed that 610 pts were enrolled from 76 study centers and randomized to Cadonilimab + chemo (n=305) or placebo + chemo (n=305) with a median follow-up of 18.69 months (range 0.0-22.7). The median OS was longer in the cadonilimab group versus the placebo group (HR 0.62 [95%CI: 0.50-0.78], mOS 15.0 vs 10.8 mo; P<0.001). OS benefits were consistently observed at all pre-specified CPS cutoffs, especially in patients with low PD-L1 expression, which still showed survival advantage. Longer PFS was observed in the cadonilimab group (HR 0.53 [95%CI: 0.44-0.65], mPFS 7.0 vs 5.3 mo; p<0.001). The ORR was 65.2% vs 48.9%, respectively, with a median DOR of 8.8 vs 4.4 months. Grade ≥3 treatment-related AEs occurred in 71.8% of pts who received cadonilimab and 60.5% of pts who received placebo; no new safety signals were observed.
It can be concluded that Cadonilimab plus chemo showed significant improvement in OS and PFS regardless of PD-L1 status and manageable safety profile, which could be a new standard first-line treatment option for advanced G/GEJ adenocarcinoma.
How to Easily View the Clinical Results Using Synapse Database?
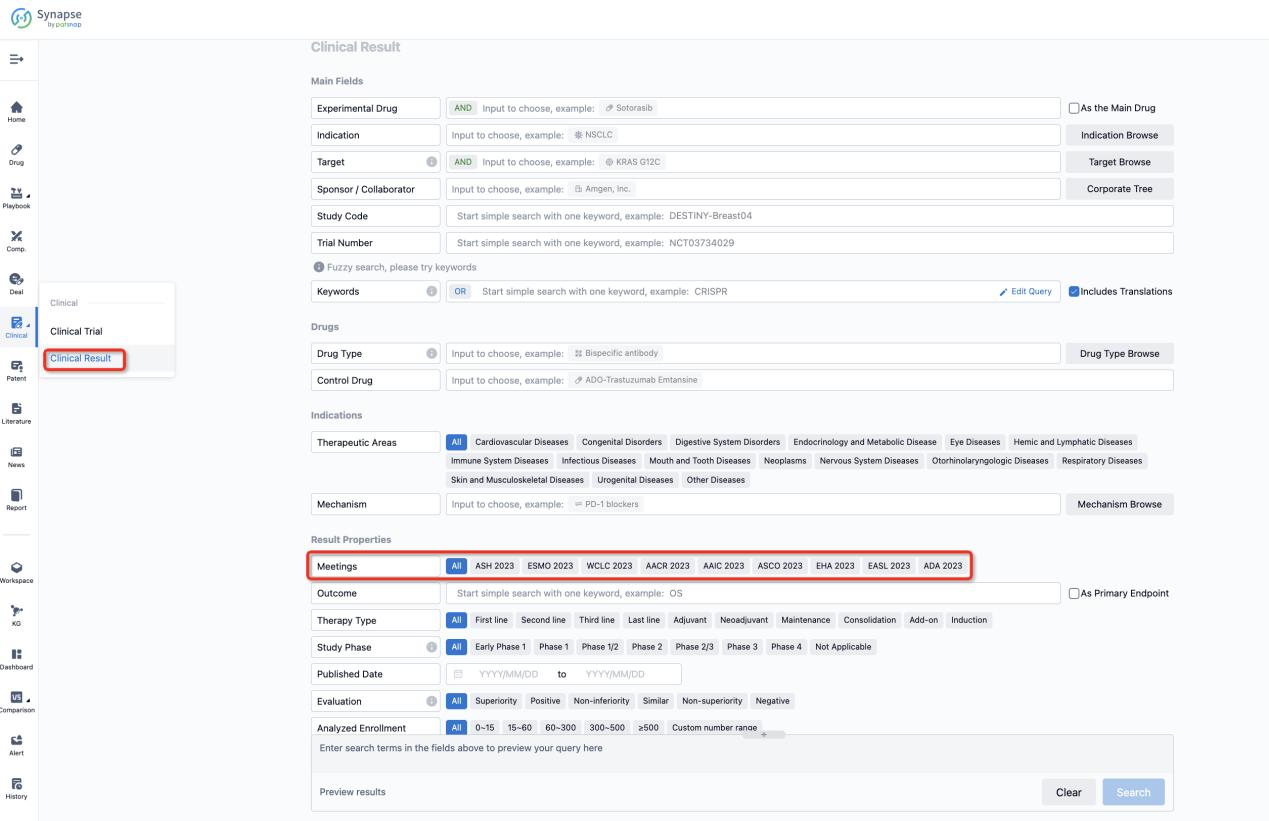
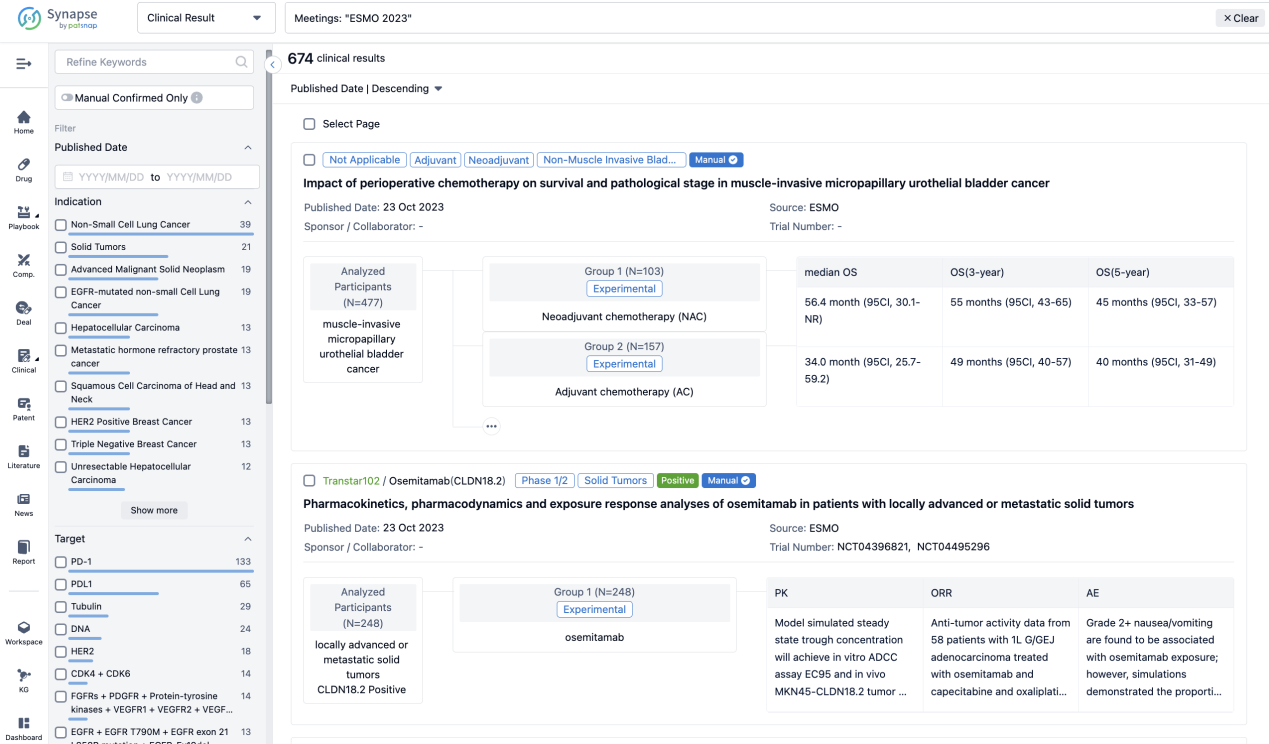
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!