EU Approves Pfizer's VELSIPITY® for Moderate to Severe Ulcerative Colitis
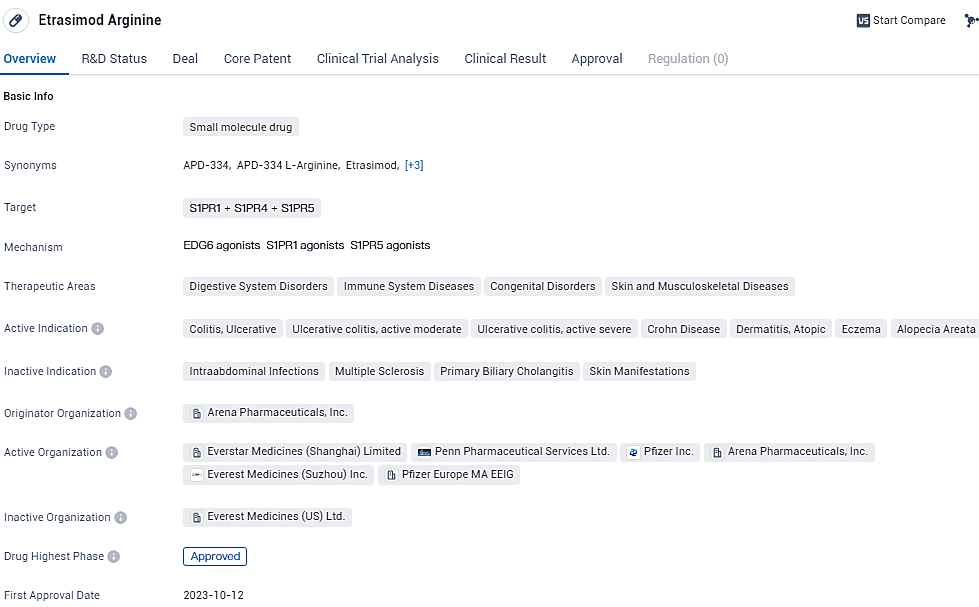
Pfizer Inc. has revealed that the European Commission has approved VELSIPITY® (etrasimod) for distribution within the European Union. This approval allows the treatment to be used for individuals aged 16 and above who are experiencing moderate to severe ulcerative colitis and have not responded well to standard treatment, have ceased responding, or cannot tolerate previous therapeutic options, including biological treatments.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Approximately 2.6 million individuals in Europe grapple with the challenges of ulcerative colitis (UC), facing a spectrum of fluctuating physical and psychological difficulties that can be severely incapacitating. Many engage in a trial-and-error process with various standard treatments in an effort to alleviate their distressing symptoms, explained Séverine Vermeire, MD, PhD, a Medicinal Professor at KU Leuven and a primary researcher in the ELEVATE Registrational Program.
Dr. Vermeire added that the sanctioning of VELSIPITY offers a new avenue for those who live with moderate to severe forms of UC and seek powerful treatments beyond traditional options, but who might be reluctant to adopt injectable biologic therapies.
VELSIPITY has received marketing clearance across all 27 European Union countries and has expanded to include Iceland, Liechtenstein, and Norway. This clearance comes on the heels of the European Medicines Agency’s Committee for Medicinal Products for Human Use endorsement in December 2023.
Previous approvals for VELSIPITY for moderate to severe UC in adult patients by the U.S. Food and Drug Administration in October 2023, as well as the Canadian authorization in January 2024 for adults with an unsatisfactory response or intolerance to conventional or other advanced therapies, pave the way for this EU authorization. Moreover, regulatory filings for VELSIPITY's usage in UC are currently under review in several other nations worldwide.
Alexandre de Germay, Pfizer's Chief International Commercial Officer and Executive Vice President, highlighted that VELSIPITY may provide an advantage for patients with UC who have not attained remission through traditional therapies. He emphasized its convenient single daily oral dose and a positive balance of benefits and risks. According to de Germay, VELSIPITY represents a promising treatment possibility, and Pfizer is committed to delivering it to eligible UC patients, starting at 16 years of age, throughout the European market.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
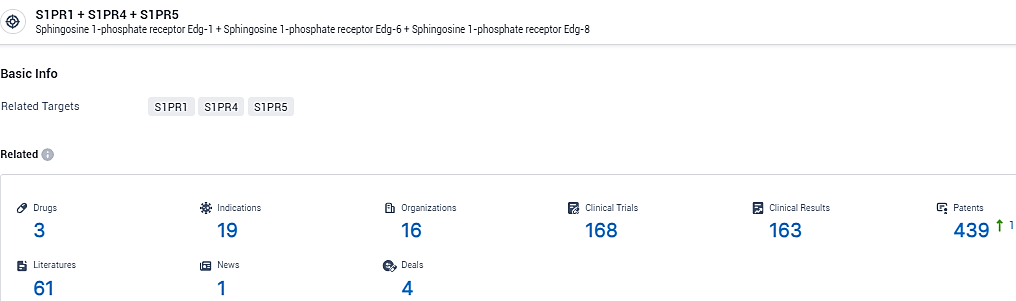
According to the data provided by the Synapse Database, As of February 23, 2024, there are 3 investigational drugs for the S1PR1 and S1PR4 and S1PR5 target, including 19 indications,16 R&D institutions involved, with related clinical trials reaching 168, and as many as 439 patents.
VELSIPITY is a once-daily, oral, sphingosine 1-phosphate (S1P) receptor modulator that selectively binds with S1P receptor subtypes 1, 4, and 5. Its approval and potential launch in the coming years will likely contribute to advancements in the treatment of digestive system disorders, immune system diseases, congenital disorders, and skin/musculoskeletal diseases.