Exploring Secretin's Revolutionary R&D Successes and its Mechanism of Action on Drug Target
Secretin's R&D Progress
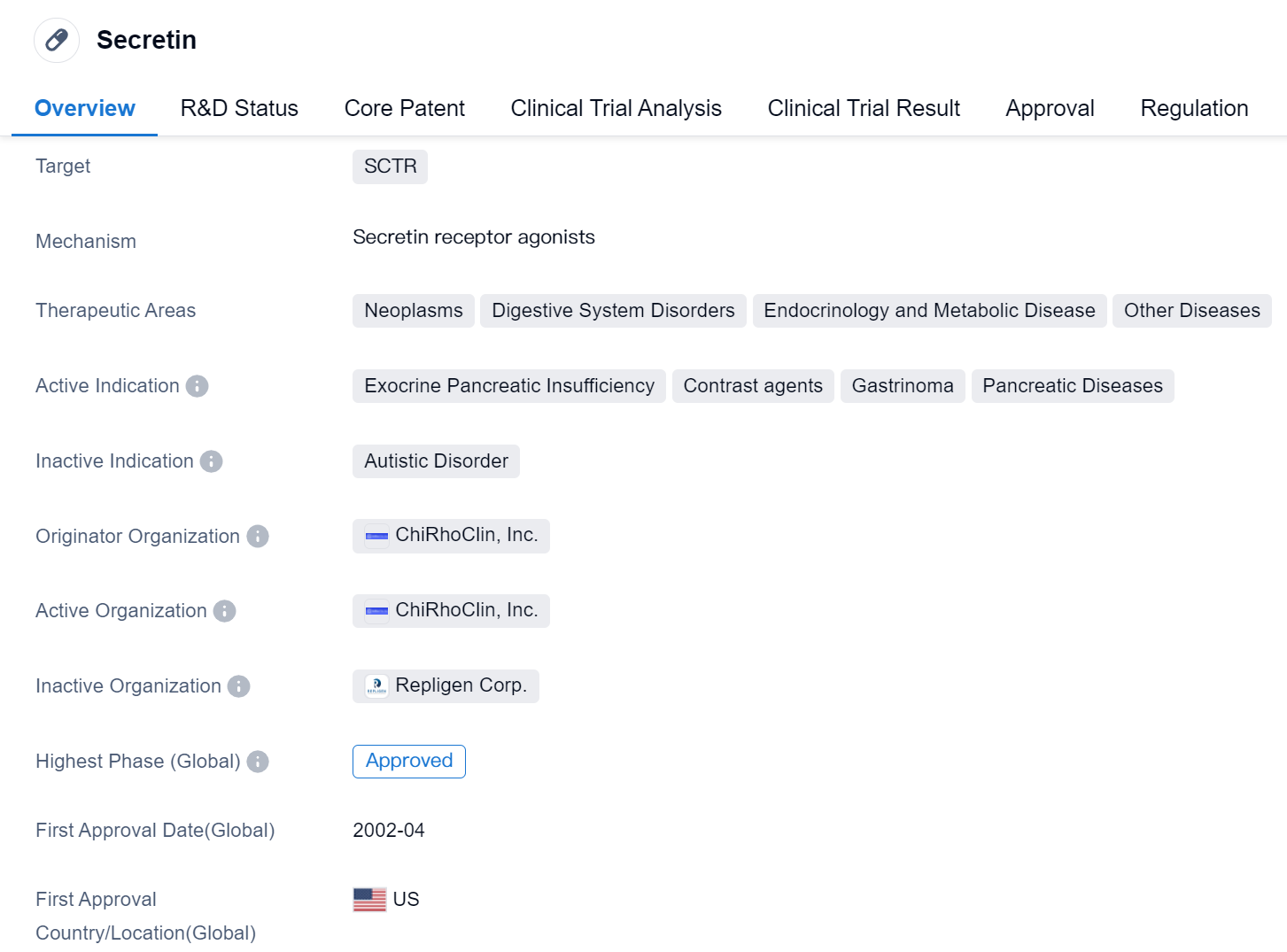
Secretin is a small molecule drug that targets the secretin receptor (SCTR). It has been approved for use in various therapeutic areas including neoplasms, digestive system disorders, endocrinology and metabolic disease, and other diseases. The drug is indicated for the treatment of exocrine pancreatic insufficiency, contrast agents, gastrinoma, and pancreatic diseases.
Secretin was developed by ChiRhoClin, Inc., an originator organization in the pharmaceutical industry. The drug has reached the highest phase of development, which is approval. It received its first approval in the United States in April 2002. Secretin is regulated as an orphan drug, indicating that it is intended to treat rare diseases or conditions.
The approval of Secretin in 2002 marked an important milestone in the treatment of various diseases related to the pancreas and digestive system. Exocrine pancreatic insufficiency, a condition characterized by the inability of the pancreas to produce enough digestive enzymes, can lead to malabsorption and other complications. Secretin has been shown to improve pancreatic function and alleviate symptoms in patients with this condition.
Additionally, Secretin is used as a contrast agent in medical imaging procedures. It helps to enhance the visibility of certain structures in the digestive system, aiding in the diagnosis of various gastrointestinal disorders. This application of Secretin has proven to be valuable in clinical practice.
Gastrinoma, a type of tumor that affects the pancreas or duodenum, can cause excessive production of gastrin, a hormone that stimulates the release of gastric acid. Secretin has been found to be effective in managing gastrinoma by inhibiting the release of gastrin and reducing gastric acid secretion.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Secretin: Secretin receptor agonists
Secretin receptor agonists are a type of drugs that bind to and activate the secretin receptors in the body. Secretin is a hormone that is naturally produced in the small intestine and plays a role in regulating various physiological processes, including the secretion of pancreatic enzymes and bicarbonate.
From a biomedical perspective, secretin receptor agonists can be used to stimulate the secretion of pancreatic enzymes and bicarbonate, which can be beneficial in certain medical conditions. For example, in patients with pancreatic insufficiency, where the pancreas does not produce enough digestive enzymes, secretin receptor agonists can help enhance the release of enzymes and improve digestion.
Additionally, secretin receptor agonists have also been studied for their potential therapeutic effects in other conditions, such as autism spectrum disorder and gastrointestinal disorders. Research suggests that secretin receptor agonists may modulate neurotransmitter systems and improve social behavior and gastrointestinal symptoms in individuals with autism.
In summary, secretin receptor agonists are drugs that activate the secretin receptors in the body, leading to various physiological effects. They can be used to stimulate pancreatic enzyme secretion and have potential therapeutic applications in conditions like pancreatic insufficiency and autism spectrum disorder.
Drug Target R&D Trends for Secretin
The SCTR, or Secretin receptor, plays a crucial role in the human body by regulating various physiological processes. It is primarily found in the pancreas and the gastrointestinal tract. When secretin, a hormone, binds to the SCTR, it stimulates the release of bicarbonate ions from the pancreas, aiding in the neutralization of stomach acid. This receptor also influences the secretion of digestive enzymes and regulates the movement of food through the digestive system. Additionally, the SCTR has been implicated in other functions such as cell growth and differentiation. Understanding the role of SCTR is essential for developing therapeutic interventions targeting digestive disorders and related conditions.
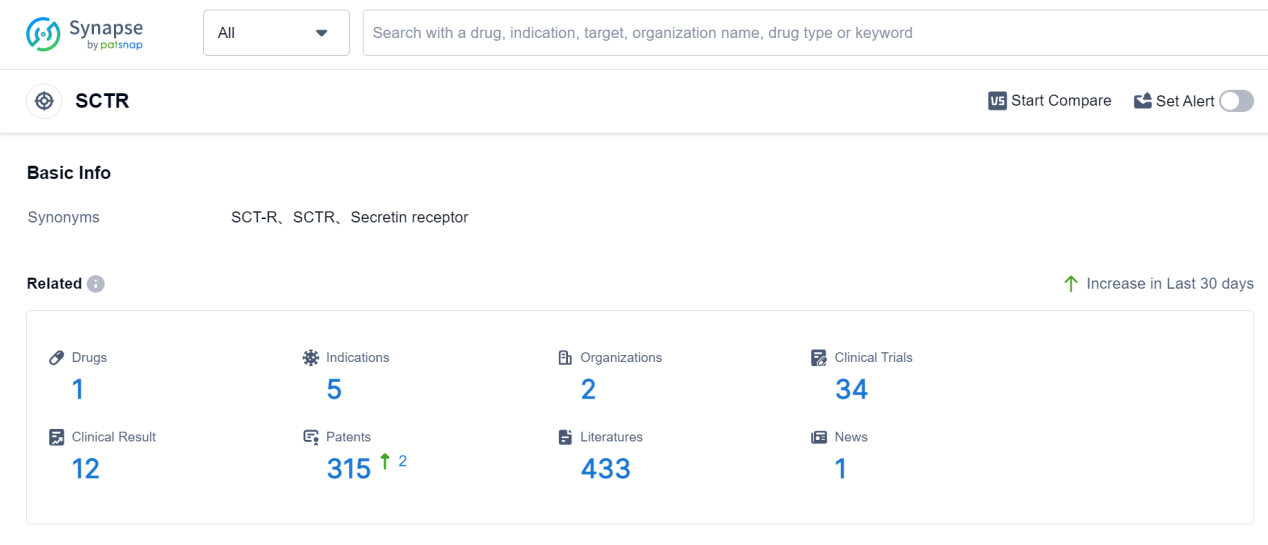
According to Patsnap Synapse, as of 7 Sep 2023, there are a total of 1 SCTR drugs worldwide, from 2 organizations, covering 5 indications, and conducting 34 clinical trials.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Secretin has demonstrated its efficacy and safety in the treatment of exocrine pancreatic insufficiency, as a contrast agent, and in managing gastrinoma. Its approval as an orphan drug highlights its importance in addressing rare diseases or conditions. The development and approval of Secretin have contributed significantly to the field of biomedicine, providing healthcare professionals with an effective therapeutic option for various pancreatic and digestive system disorders.