Decoding Rimegepant Sulfate: A Comprehensive Study of its R&D Trends and Mechanism on Drug Target
Rimegepant Sulfate's R&D Progress
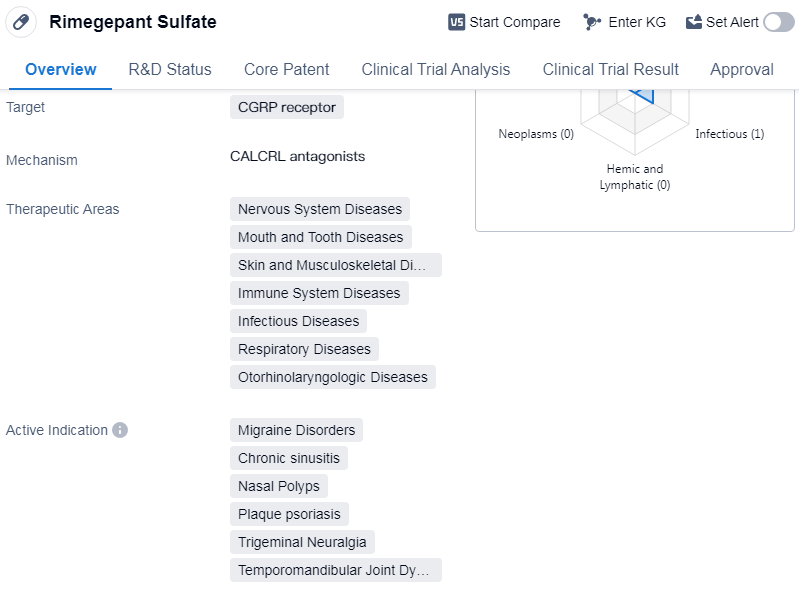
Rimegepant Sulfate is a small molecule drug that targets the calcitonin gene-related peptide (CGRP) receptor. It has been developed by Bristol Myers Squibb Co. and has received approval for use in the treatment of various diseases. The drug falls under the therapeutic areas of nervous system diseases, mouth and tooth diseases, skin and musculoskeletal diseases, immune system diseases, infectious diseases, respiratory diseases, andotorhinolaryngologic diseases.
Rimegepant Sulfate has shown efficacy in treating several indications, including migraine disorders, chronic sinusitis, nasal polyps, plaque psoriasis, trigeminal neuralgia, and temporomandibular joint dysfunction syndrome. These conditions are often associated with significant discomfort and can negatively impact patients' quality of life.
Rimegepant Sulfate has undergone rigorous testing and reached the highest phase of development, receiving global approval. It was first approved in the United States in February 2020, marking a significant milestone for the pharmaceutical industry.
In China, Rimegepant Sulfate is currently in the NDA/BLA phase, indicating that it is undergoing the process of seeking approval for use in the country. This suggests that the drug may soon be available to patients in China, providing them with a potential treatment option for the indicated conditions.
The approval of Rimegepant Sulfate highlights the potential of small molecule drugs in targeting specific receptors and addressing various diseases. The drug's ability to target the CGRP receptor makes it a promising option for the treatment of conditions related to the nervous system, mouth and tooth, skin and musculoskeletal system, immune system, infectious diseases, respiratory diseases, and otorhinolaryngologic diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Rimegepant Sulfate: CALCRL Antagonists
Calcitonin receptor-like(CALCRL) antagonists are a type of drugs that act as antagonists to the CALCRL receptor. The CALCRL receptor, also known as the calcitonin receptor-like receptor, is a G protein-coupled receptor (GPCR) that is involved in various physiological processes, including cardiovascular function, inflammation, and pain modulation.
By acting as antagonists to the CALCRL receptor, CALCRL antagonists block the binding of specific ligands to the receptor, thereby inhibiting its activation and downstream signaling pathways. This modulation of the CALCRL receptor activity can have therapeutic implications in different biomedical contexts.
For example, in the field of cardiovascular medicine, CALCRL antagonists may be explored as potential treatments for conditions such as hypertension or heart failure. By blocking the CALCRL receptor, these antagonists can help regulate blood pressure and improve cardiac function.
In summary, CALCRL antagonists are drugs that inhibit the activity of the CALCRL receptor, which has implications for various physiological processes. Their use as therapeutic agents can be explored in different biomedical contexts, particularly in cardiovascular medicine.
Drug Target R&D Trends for Rimegepant Sulfate
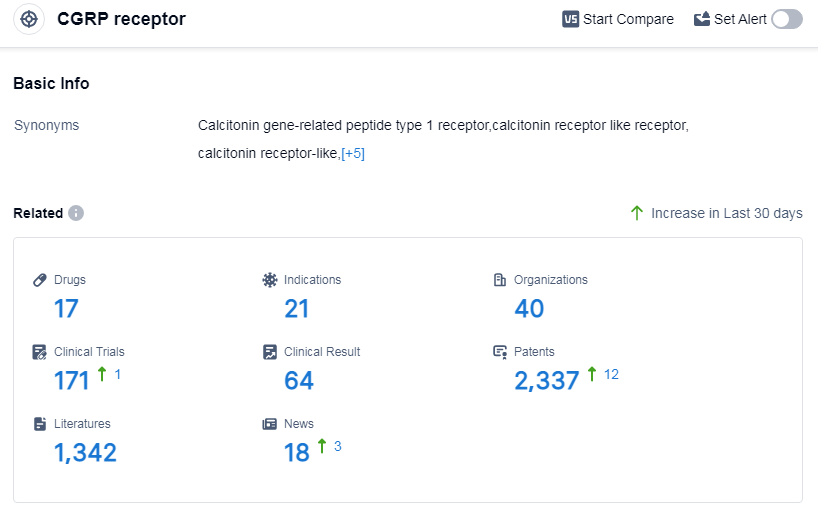
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 17 CGRP receptor drugs worldwide, from 40 organizations, covering 21 indications, and conducting 171 clinical trials.
The analysis of the target CGRP receptor in the pharmaceutical industry reveals that Pfizer Inc., Amgen, Inc., and Novartis AG are the companies growing fastest under this target. Migraine disorders have the highest number of approved drugs, and small molecule drugs and monoclonal antibodies are progressing most rapidly. The United States is leading in terms of approved drugs, while China is making progress in the development of drugs targeting the CGRP receptor. The competitive landscape for the target CGRP receptor is dynamic, with multiple companies and drug types involved in R&D efforts. The future development of the target CGRP receptor holds promise for the treatment of various indications and the advancement of innovative drugs.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Rimegepant Sulfate represents a significant advancement in biomedicine, offering hope for patients suffering from debilitating conditions. Its approval in the United States and ongoing development in China demonstrate this drug's global interest and potential impact in improving patient outcomes and quality of life.