Eisai Presents E2814's Potential to Block Tau Propagation at 17th CTAD Alzheimer's Conference
Eisai Co. Ltd, based in Tokyo and led by CEO Haruo Naito, revealed that new data regarding the anti-MTBR (microtubule binding region) tau antibody E2814 were shared at the 17th Clinical Trials on Alzheimer’s Disease (CTAD) conference, which took place in Madrid, Spain, as well as online. Additionally, Eisai has commenced a Phase II trial (Study 202) of E2814 targeting sporadic early Alzheimer’s Disease (AD).
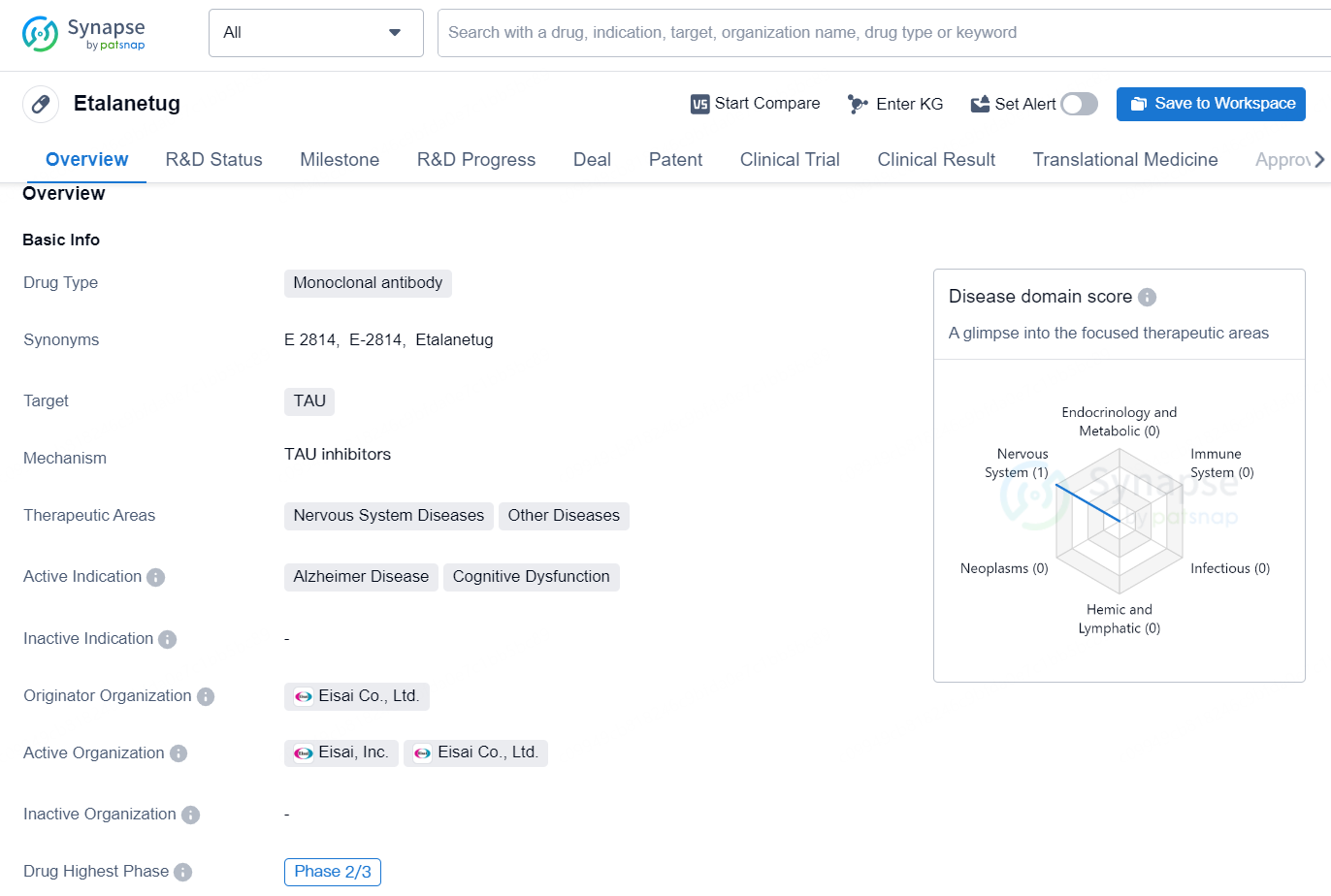
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
E2814 is a novel investigational antibody targeting the microtubule binding region (MTBR) of tau, aimed at treating tau-related conditions. In Alzheimer's disease (AD) patients, the presence of neurofibrillary tangles (NFT) is a significant pathological feature, which are thought to propagate along synaptically connected pathways in the brain, leading to the tau propagation hypothesis. It is believed that the spread of tau pathology occurs through specific tau species that include MTBR, thereby facilitating the transfer of tau-related damage to various brain regions crucial for cognitive functioning.
Eisai initiated a Phase I/II clinical trial (Study 103, NCT04971733) in June 2021, involving 7 participants affected by Dominantly Inherited Alzheimer’s Disease (DIAD), to assess the safety and tolerability of the anti-MTBR tau antibody E2814. The primary focus of this study was to evaluate how effectively E2814 engages with MTBR-tau species present in cerebrospinal fluid (CSF) of DIAD patients. Additionally, pharmacodynamic assessments were conducted using multiple biomarkers indicative of AD tau pathology.
In the trial, DIAD patients exhibiting clinical symptoms received E2814 for a duration of 12 to 24 months. Reference data for biomarkers was derived from the Dominantly Inherited Alzheimer Network Observational Study (DIAN-obs), which serves as an observational cohort for DIAD, to analyze changes during E2814 treatment.
When compared to DIAN-obs reference data, patients treated with E2814 exhibited approximately 75% and 50% reductions in CSF levels of MTBR-tau243 and p-tau217, respectively, which are indicative of tau pathophysiology. Moreover, assessments via tau PET imaging revealed stabilization or a trend toward reduced tau accumulation in the brains of DIAD patients receiving E2814. These findings imply that E2814 may inhibit tau propagation and reduce the accumulation of tau aggregates in the brains of DIAD patients. Further investigation is planned through the ongoing Phase II/III Tau NexGen trial (NCT05269394) focusing on DIAD patients, as well as the Phase II 202 study (NCT06602258) involving sporadic early Alzheimer’s disease patients.
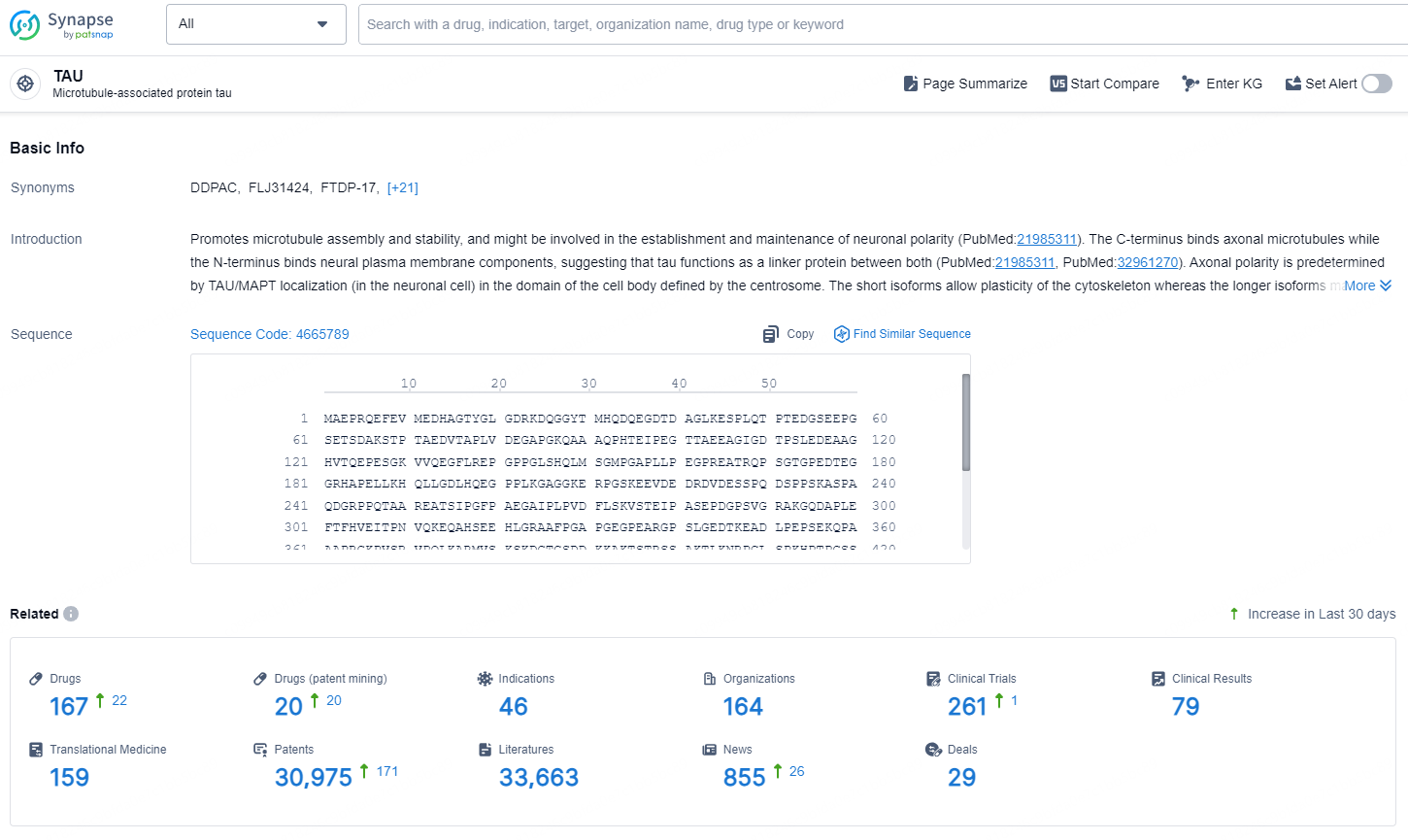
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 31, 2024, there are 167 investigational drugs for the TAU target, including 20 indications, 46 R&D institutions involved, with related clinical trials reaching 164, and as many as 30975 patents.
Etalanercept is a monoclonal antibody drug that targets TAU, a protein associated with various nervous system diseases and cognitive dysfunction. The therapeutic areas for this drug include nervous system diseases and other diseases, with a specific focus on Alzheimer's disease and cognitive dysfunction. The drug is being developed by Eisai Co., Ltd., a Japanese pharmaceutical company.