ELAHERE® Demonstrates Advantage in Overall and Progression-Free Survival in Ovarian Cancer
The leading company in the burgeoning ADC cancer treatment, ImmunoGen Inc., reported results from two subset evaluations of the MIRASOL Phase 3 validation trial. This trial assessed the safety and efficiency of ELAHERE®(mirvetuximab soravtansine-gynx) while comparing it to chemotherapy in patients affected by ovarian cancer resistant to platinum and positive for the folate receptor alpha.
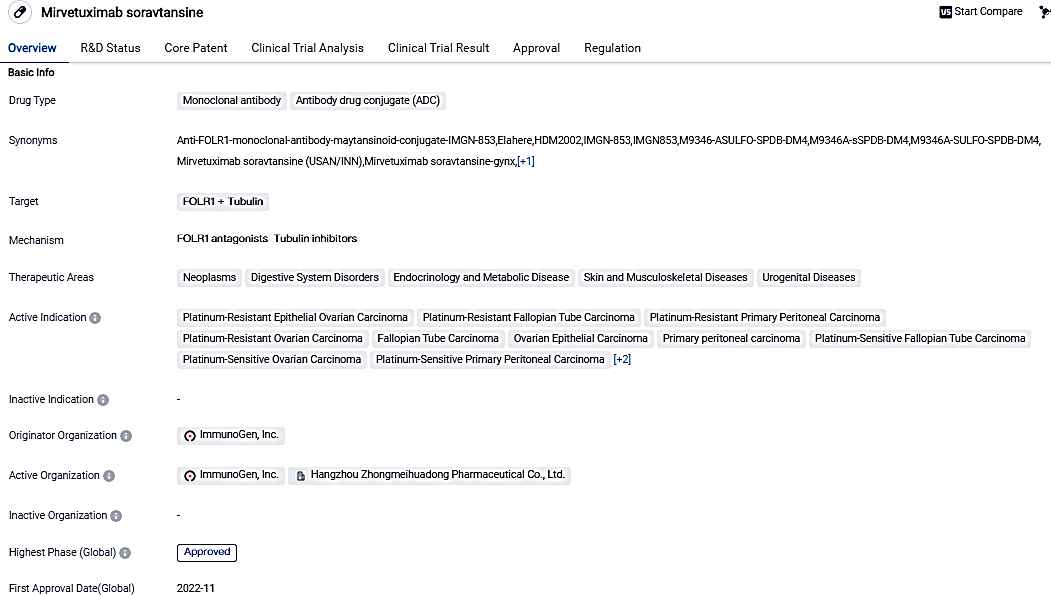
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"In line with the MIRASOL results where we saw dominance over all effectiveness benchmarks, this subgroup analysis indicates that the progression-free survival, objective response rates, and overall survival enhancements recorded in the general study group are consistent irrespective of the number of previous treatment cycles," stated Toon Van Gorp, Professor of Gynaecological Oncology at Leuven University.
Toon Van Gorp continued, “Importantly, the benefit seen with ELAHERE in patients treated with a prior PARP inhibitor is particularly encouraging, as it has been shown that PARP inhibitors have a potential negative impact on the efficacy of subsequent chemotherapies.
These new data being presented at ESGO, including a consistent safety and tolerability profile, provide valuable insights for physicians into ELAHERE’s broad and meaningful benefit compared to chemotherapy and further position ELAHERE to become the new standard of care for patients with FRα-positive PROC.”
Moreover, Van Gorp added, "The particular positive impact of ELAHERE on patients who have previously undergone treatment with a PARP inhibitor is significant, given the potential for PARP inhibitors to adversely affect the success of future chemotherapy treatments. These findings including safety and tolerability consistency, offer valuable information to doctors that ELAHERE holds over chemotherapy and corroborate ELAHERE's potential to set a new standard care for patients with FRα-positive PROC."
The MIRASOL investigation is a Phase 3 study in which ELAHERE was pitted against a single-agent chemotherapy chosen by the investigator. Stratification of patients was based on the quantity of prior treatment regimens and IC chemotherapy with the most common choice being paclitaxel (41%), followed by PLD (36%) and topotecan (23%). Previous treatments consisted of bevacizumab in 62% of patients; while 55% received a former PARP inhibitor.
ELAHERE (mirvetuximab soravtansine-gynx) represents a first-in-class ADC consisting of a folate receptor alpha-binding antibody, detachable linker, and the toxophore DM4, a powerful tubulin inhibitor developed to eradicate the targeted cancer cells.
ELAHERE® is intended for the management of adult patients suffering from folate receptor-alpha (FRα) positive, platinum-resistant epithelial ovarian, fallopian tube or primary peritoneal cancer, who have undergone between one to three systematic treatment protocols. Patient selection for the treatment relies on an FDA-endorsed test.
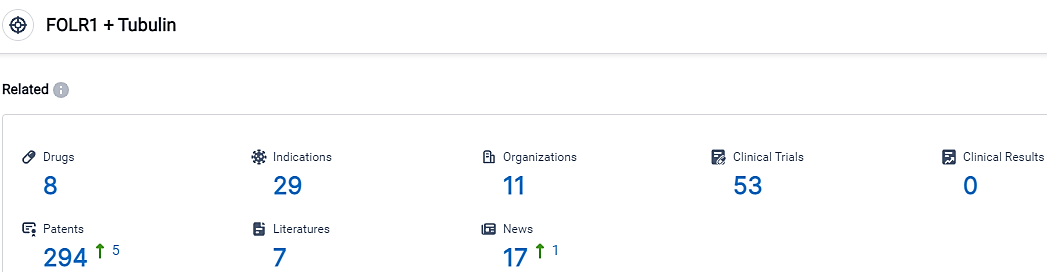
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of October 7, 2023, there are 8 investigational drugs for the FOLR1 and Tubulin target, including 29 indications,11 R&D institutions involved, with related clinical trials reaching 53,and as many as 294 patents.
The approval of Mirvetuximab soravtansine is expected to provide a new treatment option for patients with platinum-resistant ovarian and fallopian tube carcinomas, as well as other related cancers. The drug's targeted approach and potential efficacy in multiple therapeutic areas make it a promising addition to the field of biomedicine. Further studies and clinical trials will be conducted to evaluate its long-term safety and effectiveness.