MARIPOSA Phase 3 study hits key target, showing improved effectiveness of RYBREVANT® and Lazertinib versus Osimertinib for EGFR-Mut patients
The Janssen Pharmaceutical Companies of Johnson & Johnson declared favorable preliminary findings from the MARIPOSA Phase 3 trial. The trial assessed RYBREVANT® (amivantamab-vmjw), a bispecific antibody that targets the epidermal growth factor receptor (EGFR) and the mesenchymal-epithelial transition (MET), in conjunction with lazertinib, an orally-administered third-generation EGFR tyrosine kinase inhibitor. This combination was compared to osimertinib as an initial therapy in subjects with non-small cell lung cancer featuring locally advanced or metastatic EGFR mutations.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The Phase 3 MARIPOSA trial achieved its main goal by showing a notable and clinically relevant improvement in PFS in patients receiving RYBREVANT® plus lazertinib, as compared to osimertinib. The safety profile of RYBREVANT® and lazertinib showed alignment with the previously published data on the combination.
A planned interim overall survival review, a pattern was seen that favored the RYBREVANT® and lazertinib combo over osimertinib. Patients in the trial will continue to be monitored for OS analyses to determine the significance statistically and clinically.
Peter Lebowitz, M.D., Ph.D., Global Therapeutic Area Head, Oncology, Janssen Research & Development, LLC, commented on the positive preliminary results from the MARIPOSA trial. He stated they underline the potential for the RYBREVANT and lazertinib combo to become a future standard treatment for frontline EGFR-mutated non-small cell lung cancer. He said the regimen of RYBREVANT and lazertinib obstructs crucial oncogenic driver pathways and stimulates the immune system, which helps fight the disease in various ways.
As Alexander Spira†, M.D., Ph.D., FACP, Director, Virginia Cancer Specialists Research Institute, and study investigator noted, EGFR TKIs were historically used as the first line of treatment for EGFR-mutated non-small cell lung cancer patients. However, resistance and disease progression were commonly seen when used as monotherapy. As such, the hopeful data from MARIPOSA highlight the potential for the RYBREVANT and lazertinib combination in revolutionizing treatment beyond TKI monotherapy.
After PAPILLON and MARIPOSA-2, MARIPOSA is the third Phase 3 study of RYBREVANT® to produce results. Plans are underway by Janssen to submit these results for presentation at an impending scientific congress, which will contain additional information on chosen secondary endpoints.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
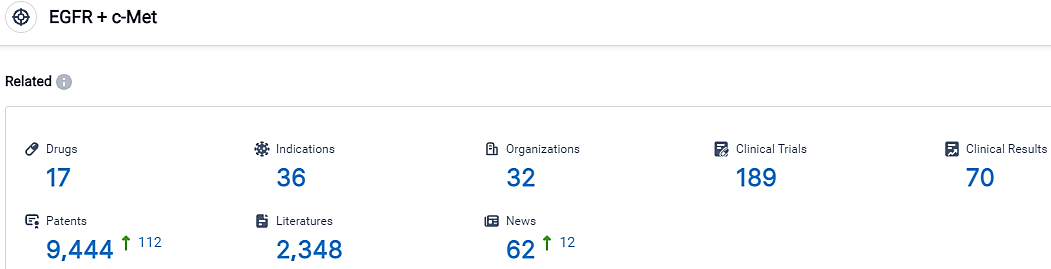
According to the data provided by the Synapse Database, As of October 7, 2023, there are 17 investigational drugs for the EGFR and c-Met target, including 36 indications,32 R&D institutions involved, with related clinical trials reaching 189,and as many as 9444 patents.
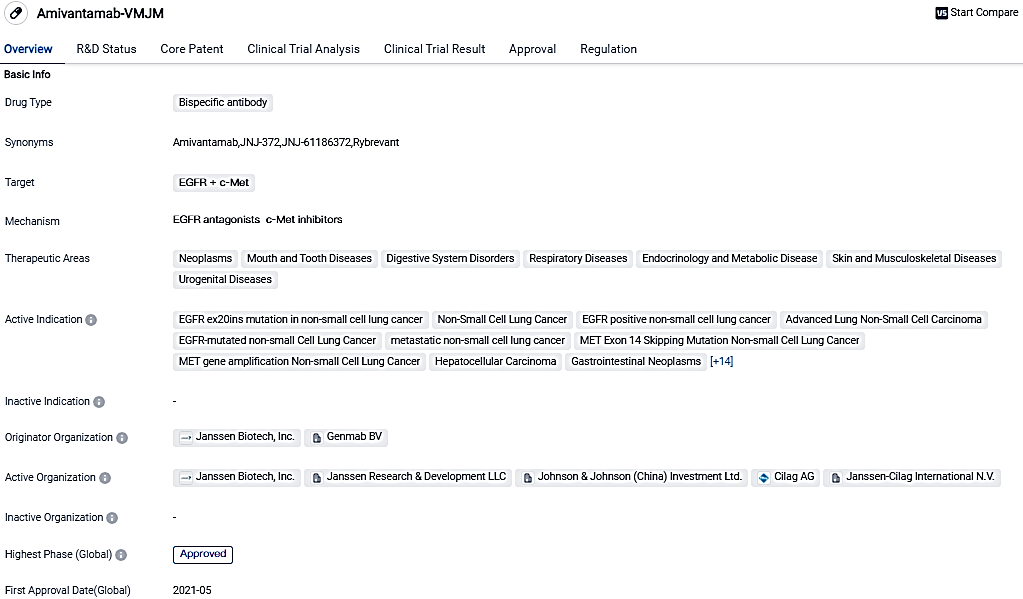
RYBREVANT®, a fully-human bispecific antibody targeting EGFR and MET with immune cell-directing activity, received accelerated approval by the U.S. FDA in May 2021 for the treatment of adult patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations, as detected by an FDA-approved test, whose disease has progressed on or after platinum-based chemotherapy. RYBREVANT® has also received approval from health authorities in Europe, as well as other markets around the world.