Gracell Biotechnologies Announces Clinical Results for FasTCAR-T GC012F in High-Risk, Multiple Myeloma
Gracell Biotechnologies Inc., an international clinical-stage bio-pharmaceutical company committed to creating ground-breaking and powerful cell therapies for curing cancer and autoimmune disease, has showcased updated long-term data from a continuing Phase 1 trial.
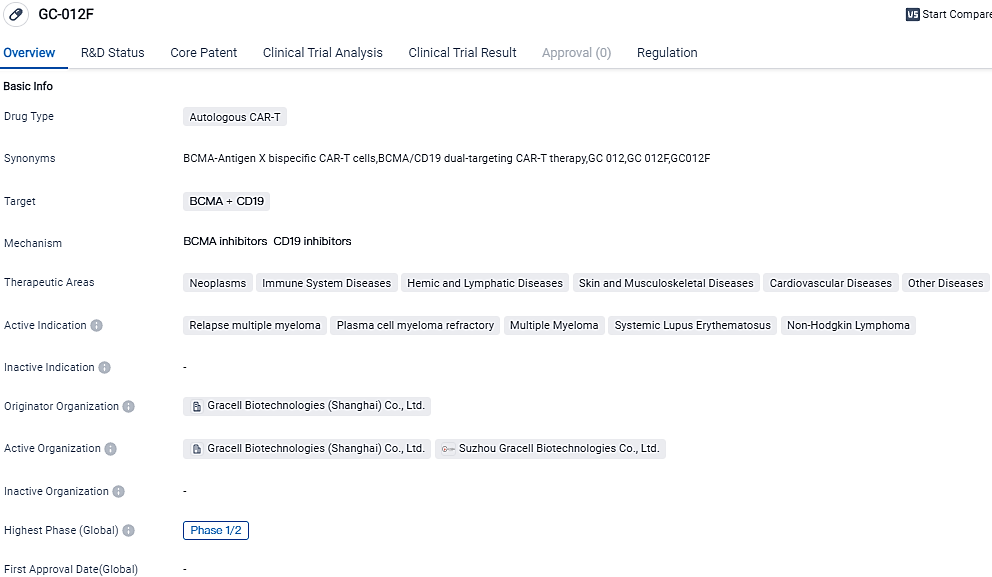
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
This trial is assessing GC012F, a candidate for autologous CAR-T treatment that simultaneously targets CD19 and the B-cell maturation antigen (BCMA). It is being tested as an initial treatment method for patients qualifiable for transplant and those recently diagnosed with high-risk multiple myeloma.
As per the data cutoff date on August 1, 2023, GC012F showed a complete 100% ORR and 100% MRD- sCR rate among 19 high-risk NDMM patients who were suitable for transplant. This data is inclusive of an extended review from the first 16 patients, whose preliminary findings were put forth at the annual conference of the American Society for Hematology in 2022, along with three more patients who were enrolled and given treatment.
"High-risk NDMM patients, especially those who are eligible for transplant, often face less than optimal results with the present standard of care. It is crucial to introduce innovative methods to confront the difficulties posed by this patient population that is challenging to treat," commented Dr. Wendy Li, the Chief Medical Officer at Gracell.
"MRD- sCR is the best possible response in treating patients with multiple myeloma. We are pleased to announce that in this particular study, all 19 NDMM patients who received treatment via GC012F attained MRD- sCR with a positive safety record. This is a strong endorsement of the incredible potential of CAR-T therapy, our unique BCMA/CD19 dual-targeting strategy, and the superior T cell fitness facilitated by our FasTCAR manufacturing technology," added Dr. Wendy Li.
GC012F is Gracell’s BCMA/CD19 dual-targeting autologous CAR-T cell therapy, powered by FasTCAR, which aims to revolutionize cancer and autoimmune disease treatments by facilitating quick, profound, and long-lasting responses, alongside an enhanced safety profile.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
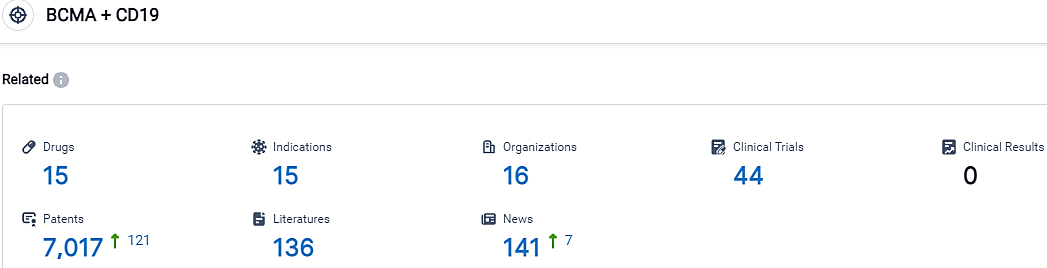
According to the data provided by the Synapse Database, As of October 7, 2023, there are 15 investigational drugs for the BCMA/CD19 target, including 15 indications,16 R&D institutions involved, with related clinical trials reaching 44,and as many as 7017 patents.
GC012F is currently being evaluated in clinical studies in multiple hematological cancers as well as autoimmune diseases, and has demonstrated a consistently strong efficacy and safety profile. Gracell has initiated a Phase 1b/2 trial evaluating GC012F for the treatment of relapsed/refractory multiple myeloma in the United States and a Phase 1/2 clinical trial in China is to be commenced imminently. Further research and development will be needed to determine the efficacy and safety of GC-012F in treating these conditions.