Interpretation of Gene Therapy Company
Gene therapy refers to the introduction of exogenous normal genes into target cells to correct or compensate for diseases caused by defective and abnormal genes, in order to achieve therapeutic purposes. This includes the application of technologies such as genetic engineering, which is the insertion of exogenous genes into appropriate recipient cells of the patient through gene transfer technology, enabling the products produced by exogenous genes to treat certain diseases. Broadly speaking, gene therapy can also include measures and new technologies for treating certain diseases at the DNA level.
The success of gene therapy relies on the use of suitable carriers to safely and effectively deliver genetic material to affected cells. This carrier can either be delivered directly to the body for absorption by a single cell, or delivered to the patient's cell samples in the lab and then returned to the body. Some viruses, due to their ability to infect cells, are often used as carriers for gene therapy. Some types of viruses, such as retroviruses, incorporate genetic material, including new genes, into cell chromosomes. Other viruses, such as adenoviruses, transfer their DNA into the cell nucleus, but do not integrate with the cell chromosomes. In addition, viruses can also deliver gene editing tools into the cell nucleus.
Gene Therapy Competitive Landscape
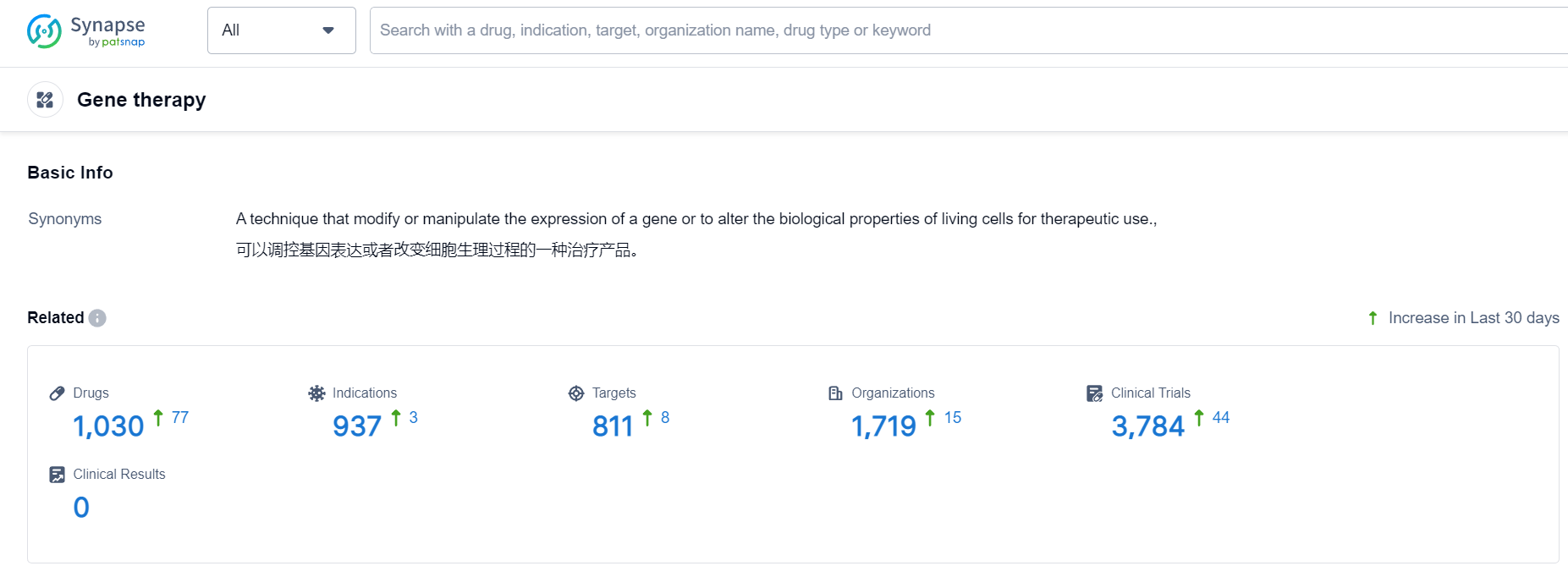
According to Patsnap Synapse, as of 9 Oct 2023, there are a total of 1030 Gene therapy drugs worldwide, from 1719 organizations, covering 811 targets, 937 indications, and conducting 3785 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, targets, organizations, clinical trials, and clinical results related to this drug type.
Approved Gene Therapy Medicinal: Beremagene geperpavec
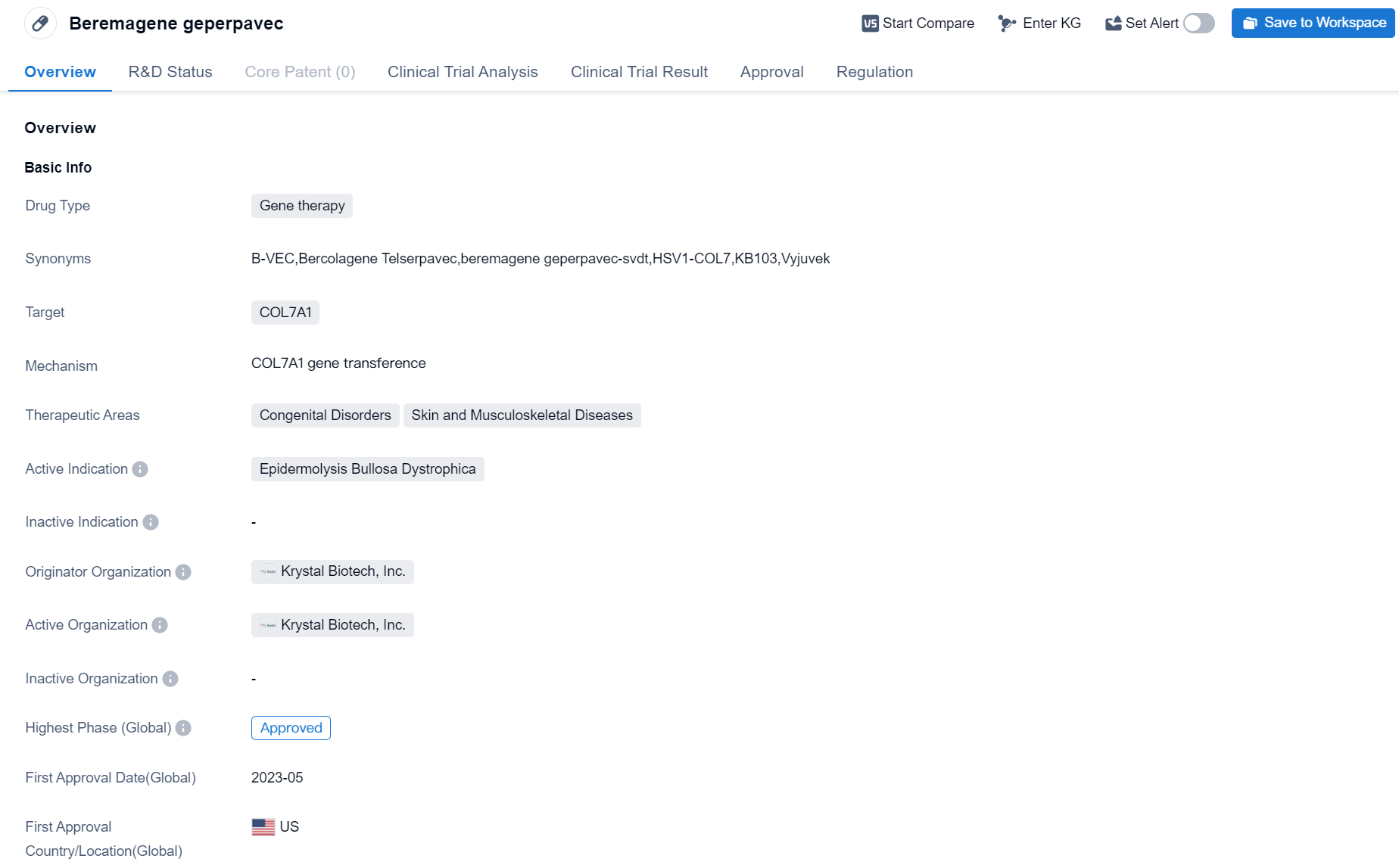
Beremagene geperpavec is a gene therapy drug developed by Krystal Biotech, Inc. It falls under the therapeutic areas of Congenital Disorders, Skin and Musculoskeletal Diseases. The drug specifically targets COL7A1, a gene associated with Epidermolysis Bullosa Dystrophica, a rare genetic disorder characterized by fragile skin and blister formation.
Beremagene geperpavec has successfully completed its highest phase of development and has received approval for use. The drug obtained its first approval in the United States in May 2023. This signifies that it has met the necessary regulatory requirements and has been deemed safe and effective for the treatment of Epidermolysis Bullosa Dystrophica.
The regulatory status of Beremagene geperpavec includes several designations that aim to expedite its development and approval process. These designations include Priority Review, Regenerative Medicine Advanced Therapy, Paediatric investigation plan, Rare Pediatric Disease, PRIME, Fast Track, and Orphan Drug. These designations highlight the significance of the drug in addressing an unmet medical need and its potential to provide therapeutic benefits to patients with Epidermolysis Bullosa Dystrophica.
As a gene therapy, Beremagene geperpavec works by introducing a functional copy of the COL7A1 gene into the patient's cells. This gene is responsible for producing a protein called collagen type VII, which is crucial for maintaining the integrity of the skin. By restoring the production of this protein, the drug aims to alleviate the symptoms associated with Epidermolysis Bullosa Dystrophica and improve the quality of life for affected individuals.
The approval of Beremagene geperpavec marks a significant milestone in the field of gene therapy and offers hope for patients suffering from Epidermolysis Bullosa Dystrophica. It represents a breakthrough in the treatment of rare genetic disorders and showcases the potential of gene therapies in addressing unmet medical needs. The drug's approval in the United States and its various regulatory designations further emphasize its importance and potential impact in the field of biomedicine.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
A Crucial Gene Therapy Company: Krystal Biotech
Krystal Biotech, Inc. is a biomedicine organization that was founded in 2015 and is located in Pennsylvania, United States. The company focuses on the development of therapeutics for various therapeutic areas.
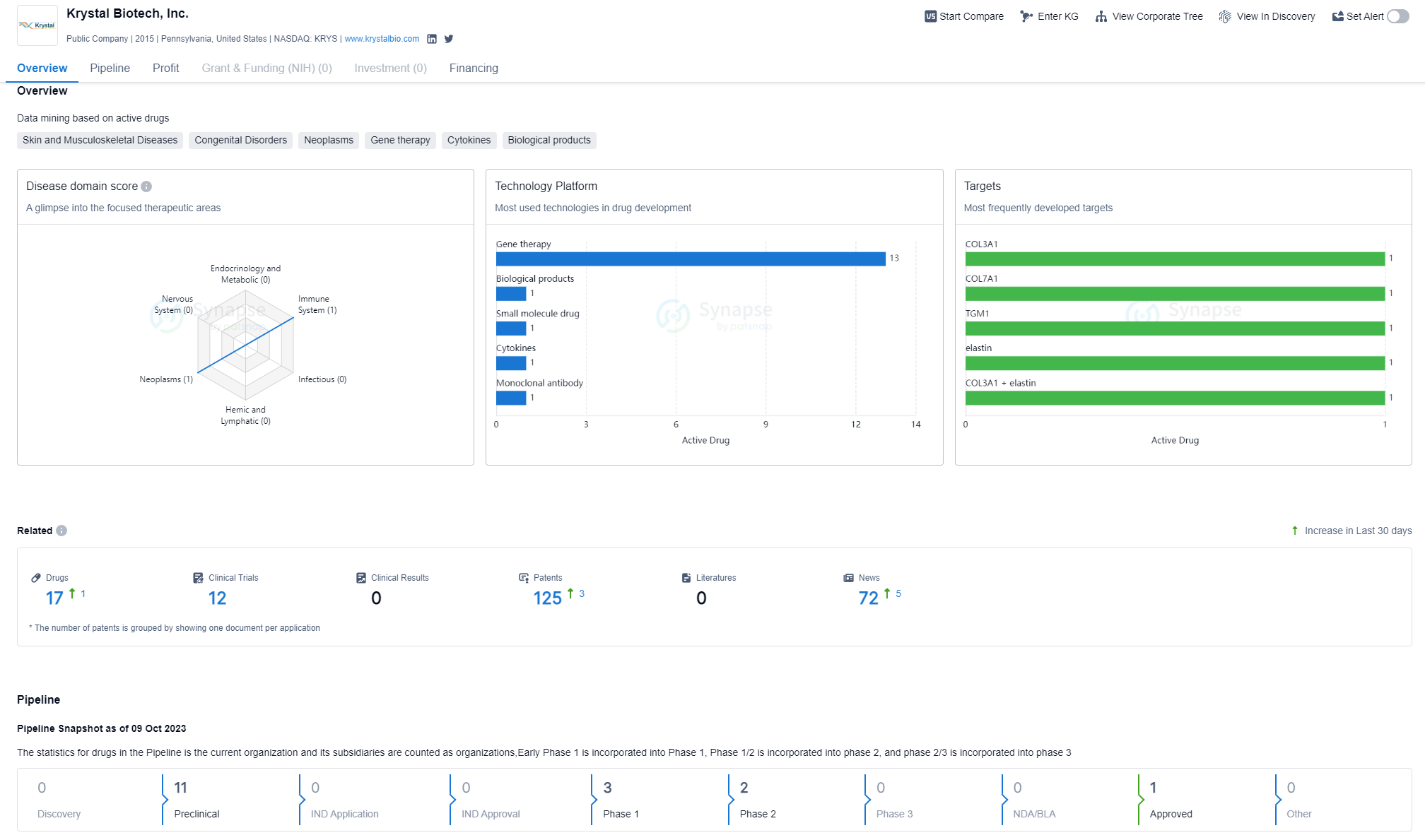
Krystal Biotech has developed drugs for a range of therapeutic areas. The highest drug count is in the field of Skin and Musculoskeletal Diseases, with 13 drugs. This indicates that the company has a strong focus on developing treatments for conditions related to the skin and musculoskeletal system. Congenital Disorders is the second highest therapeutic area, with 7 drugs. This suggests that Krystal Biotech is also dedicated to addressing genetic disorders that are present from birth. Respiratory Diseases, Digestive System Disorders, and Other Diseases have a lower drug count, with 3, 2, and 2 drugs respectively. Neoplasms and Immune System Diseases have the lowest drug count, with 1 drug each.
Each target has a drug count of 1, indicating that the company has developed drugs targeting a diverse range of proteins and molecules. Some of the targets mentioned include COL3A1, COL7A1, TGM1, elastin, COL3A1 + elastin, IL-12 + IL-2, COL1A1, CFTR, LEKTI, and A1AT. These targets are associated with various therapeutic areas, suggesting that Krystal Biotech is actively exploring different avenues for drug development.
In terms of the pipeline, as of October 9, 2023, the company has no drugs in the Discovery phase. However, it has 11 drugs in the Preclinical phase, indicating that they are undergoing testing in laboratory and animal models. There are no drugs in the IND Application or IND Approval phases, which suggests that Krystal Biotech has not yet submitted any Investigational New Drug applications to the regulatory authorities.
The company has 3 drugs in Phase 1, indicating that they have progressed to the stage of testing the safety and dosage of the drugs in a small group of healthy volunteers. Additionally, there are 2 drugs in Phase 2, which suggests that Krystal Biotech is evaluating the effectiveness and side effects of these drugs in a larger group of patients. There are no drugs in Phase 3, NDA/BLA, or Approved stages, indicating that the company's drugs are still in the earlier stages of development.
Based on the information provided, Krystal Biotech appears to be a relatively young company that is actively engaged in drug development across multiple therapeutic areas. The company has a significant focus on Skin and Musculoskeletal Diseases, with a high drug count in this area. This suggests that Krystal Biotech recognizes the importance of addressing conditions related to the skin and musculoskeletal system. The company also has a strong interest in developing treatments for Congenital Disorders, indicating a commitment to addressing genetic disorders from an early stage.
In terms of the pipeline, Krystal Biotech has a significant number of drugs in the Preclinical phase, indicating that they are actively conducting research and testing in laboratory and animal models. This suggests that the company is investing in the early stages of drug development and is focused on identifying promising candidates for further evaluation. The presence of drugs in Phase 1 and Phase 2 indicates that Krystal Biotech is progressing towards evaluating the safety and effectiveness of their drugs in human subjects.
However, it is important to note that as of the given date, Krystal Biotech does not have any drugs in the later stages of development, such as Phase 3, NDA/BLA, or Approved stages. This suggests that the company's drugs are still in the earlier stages of development and have not yet reached the point of regulatory approval or commercialization.
👇Please click on the image below to directly access the latest data (Therapeutic areas | Targets | R&D Status of Drugs | Clinical Trial | Approval status in Global countries) of this company.
In conclusion, Krystal Biotech, Inc. is a biomedicine organization that was founded in 2015 and is based in Pennsylvania, United States. The company focuses on the development of therapeutics for various therapeutic areas, with a particular emphasis on Skin and Musculoskeletal Diseases and Congenital Disorders. Krystal Biotech has developed drugs targeting a diverse range of proteins and molecules, indicating a commitment to exploring different avenues for drug development. The company's pipeline shows a significant number of drugs in the Preclinical phase, indicating active research and testing. However, it is important to note that the company's drugs are still in the earlier stages of development and have not yet reached the later stages of regulatory approval or commercialization.