Is Ansuvimab-zykl approved by the FDA?
Ansuvimab-zykl, marketed under the brand name Ebanga, is an antiviral medication used for the treatment of Zaire ebolavirus infection. Ansuvimab-zykl (Ebanga) was approved by the US Food and Drug Administration (FDA) on December 21, 2020, for the treatment of Zaire ebolavirus infection in both adults and pediatric patients. This approval marked an important milestone in the fight against Ebola virus disease, providing a new therapeutic option for those affected by this deadly virus.
Uses and Administration
Uses:
- Ansuvimab-zykl is specifically indicated for the treatment of infection caused by Zaire ebolavirus.
- It is administered in a medical facility under the direct supervision of a healthcare professional.
Administration:
- The medication is given as an intravenous (IV) infusion.
- The IV infusion must be administered slowly, taking at least one hour to complete.
- It is crucial to follow the healthcare provider's instructions carefully during administration to ensure efficacy and reduce the risk of side effects.
Precautions and Considerations
Before Using Ansuvimab-zykl:
- Patients should discuss any allergies with their doctor, including allergies to medications, foods, dyes, preservatives, or animals.
- The safety and efficacy of ansuvimab-zykl have been established for the pediatric population.
- There are no specific geriatric-related problems documented to date.
Breastfeeding:
- There are no adequate studies to determine infant risk when using ansuvimab-zykl during breastfeeding. Weigh the potential benefits against the potential risks before taking this medication while breastfeeding.
Interactions:
- Ansuvimab-zykl may interact with live vaccines, which are generally not recommended during treatment.
- Patients should avoid receiving live vaccines and should not be in close contact with individuals who have recently received live vaccines due to the risk of virus transmission.
Side Effects
Common Side Effects:
- Injection site reactions (pain, redness, swelling)
- Diarrhea
Serious Side Effects:
- Back pain, blurred vision, chest tightness, chills, confusion
- Dizziness, faintness, or lightheadedness when standing
- Fast, pounding, or irregular heartbeat, fever, flushing
- Headache, nausea, vomiting, rapid shallow breathing
- Sweating, trouble breathing, unusual tiredness or weakness
If severe side effects occur, patients should seek medical attention immediately.
Conclusion
This approval provides a critical tool in the management of Ebola virus disease, offering hope to those affected by this life-threatening condition. As with any medication, it is important to follow the prescribed instructions and consult healthcare providers with any concerns regarding its use.
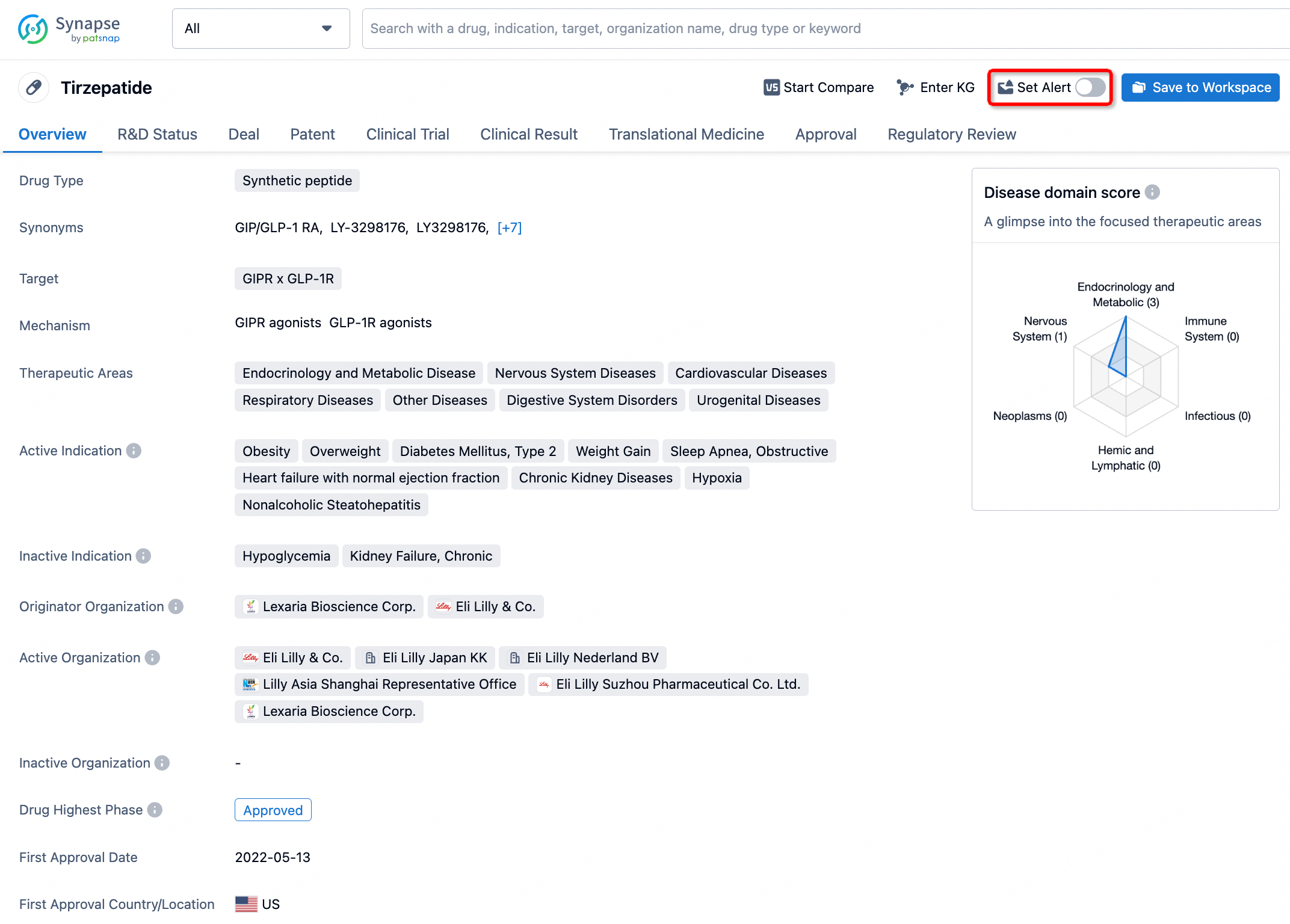
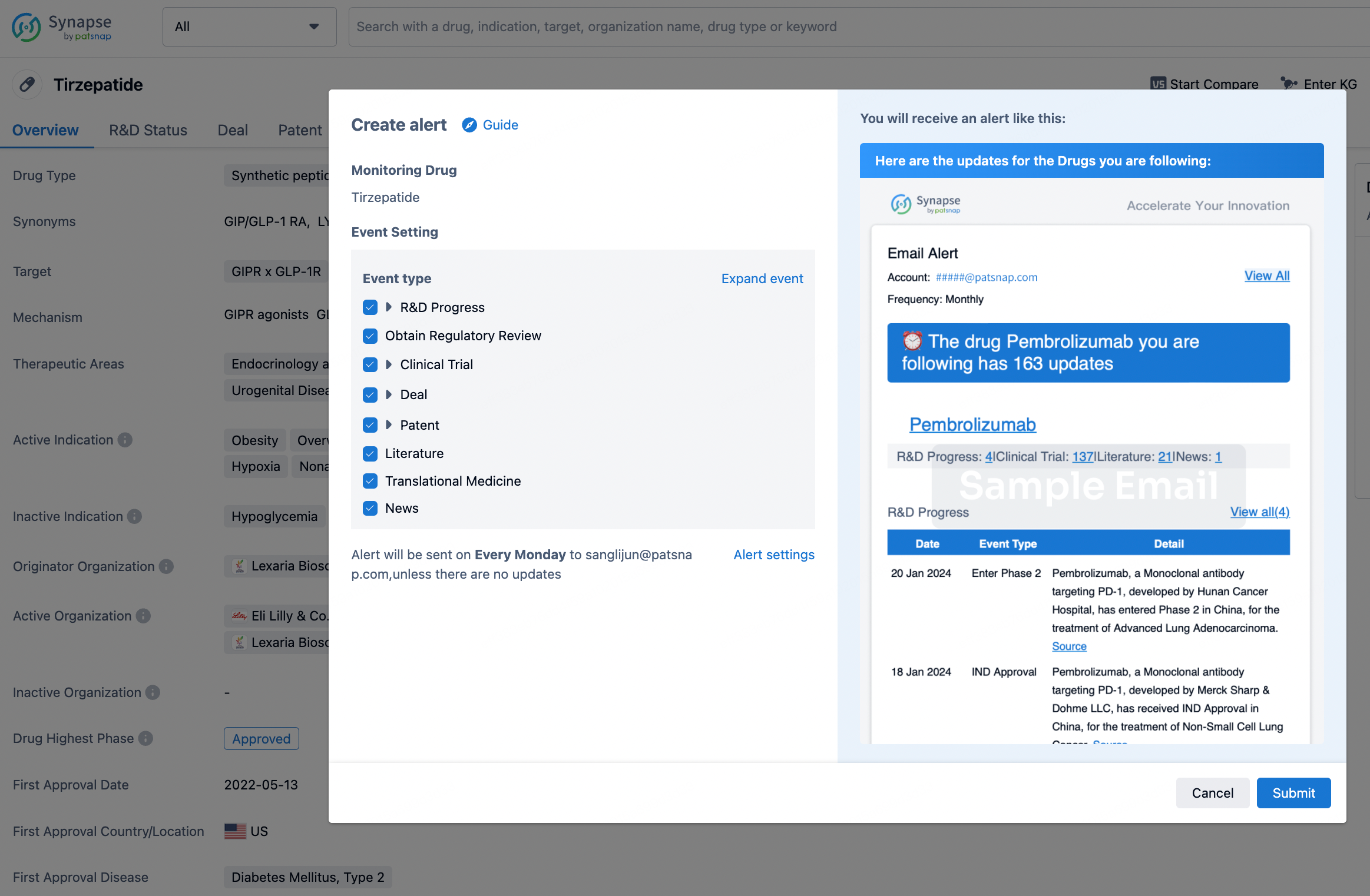
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!