European Commission Approves Pierre Fabre's BRAFTOVI® and MEKTOVI® Combo for Advanced BRAFV600E-Mutant NSCLC Treatment
Pierre Fabre Laboratories has announced that the European Commission (EC) has granted approval for the use of BRAFTOVI® (encorafenib) in conjunction with MEKTOVI® (binimetinib) for adult patients suffering from advanced non-small cell lung cancer (NSCLC) harboring a BRAFV600E mutation. This decision follows data from the Phase II PHAROS trial, an international, open-label, multicenter, non-randomized study designed to assess the efficacy and safety of the BRAFTOVI® and MEKTOVI® combination in both treatment-naïve and previously treated patients with BRAFV600E mutant metastatic NSCLC.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
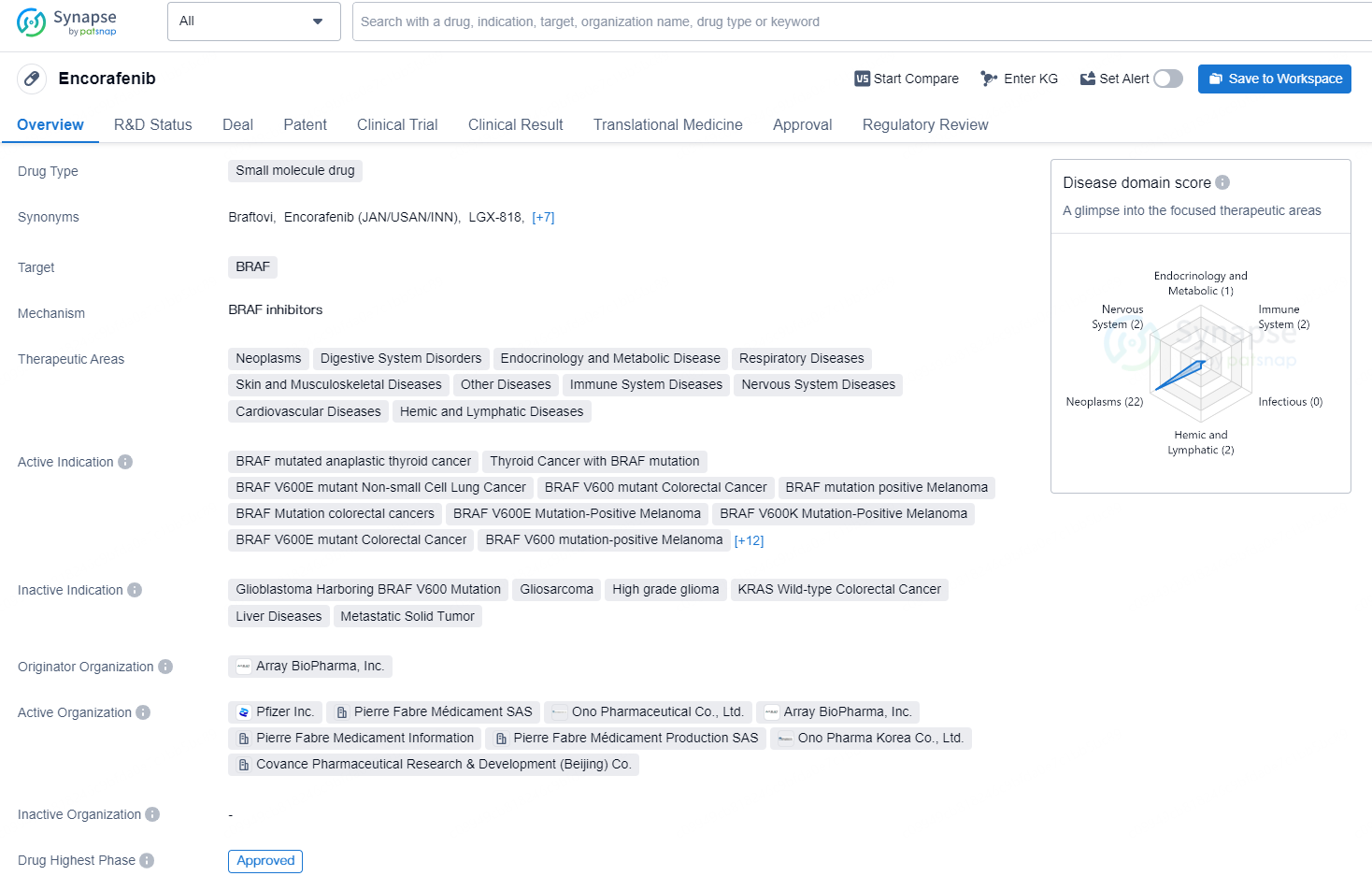
Pierre Fabre Laboratories has gained European Commission approval for BRAFTOVI® (encorafenib) in conjunction with MEKTOVI® (binimetinib) for treating adult patients with advanced non-small cell lung cancer (NSCLC) harboring a BRAFV600E mutation.
Eric Ducournau, CEO of Pierre Fabre Laboratories, commented, "We are excited to expand the use of BRAFTOVI® (encorafenib) in combination with MEKTOVI® (binimetinib) to adult patients in Europe who have advanced NSCLC with a BRAFV600E mutation. Given the limited targeted treatment options available for BRAFV600E mutant NSCLC patients, this approval is a significant advancement as it provides an additional effective targeted therapy."
The European Commission's decision follows a positive recommendation from the Committee for Medicinal Products for Human Use (CHMP) on July 25 and is supported by results from the Phase II PHAROS trial. At the primary analysis cut-off on September 22, 2022, the trial achieved its primary endpoint, indicated by the objective response rate (ORR) assessed through independent radiology review (IRR). In the treatment-naïve group (n=59), the ORR was 75% (95% CI: 62, 85), including 15% complete responses (CRs) and 59% partial responses (PRs). Updated data with an additional 10-month follow-up revealed that 64% of patients sustained a response for a minimum of 12 months, with a median duration of response (mDOR) determined by IRR at 40 months (95% CI: 23.1, not estimable [NE]).
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
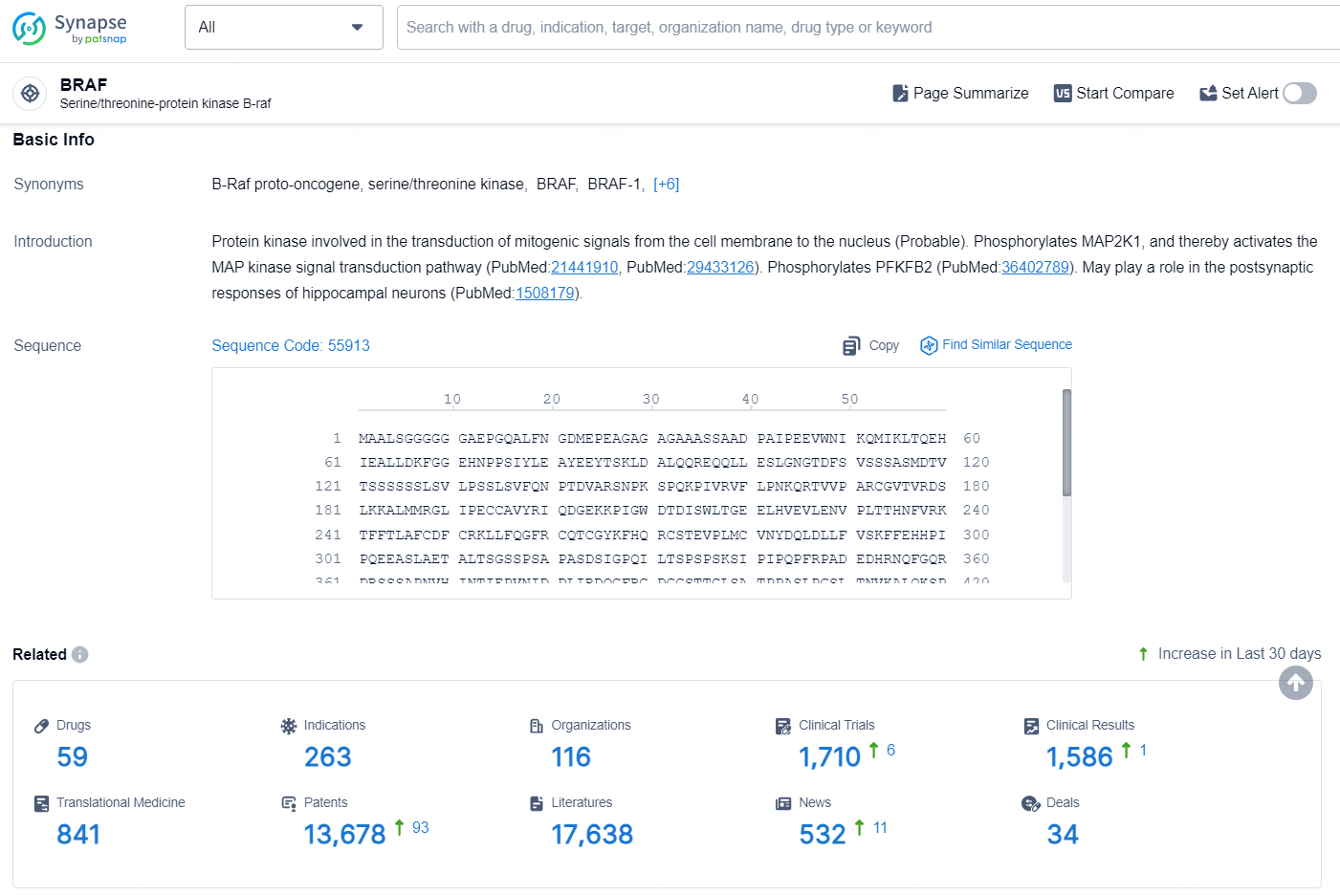
According to the data provided by the Synapse Database, As of September 3, 2024, there are 59 investigational drugs for the BRAF targets, including 263 indications, 116 R&D institutions involved, with related clinical trials reaching 1710, and as many as 13678 patents.
Encorafenib is a small molecule drug that targets the protein BRAF. It is approved for the treatment of several types of cancers, including anaplastic thyroid cancer, non-small cell lung cancer, colorectal cancer, melanoma, brain cancer, and plasma cell myeloma refractory, among others. The drug is indicated for tumors with specific BRAF mutations and has shown efficacy in treating advanced and metastatic forms of these malignancies.