European Commission Authorizes Roche's Alecensa as First Targeted Adjuvant Therapy for Early-Stage ALK-Positive Lung Cancer
Roche revealed that the European Commission has granted approval for Alecensa® (alectinib) monotherapy as an adjuvant treatment post-tumor resection. This approval is specifically for adult patients with anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer who are at a high risk of recurrence.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.

Data obtained from the Phase III ALINA trial, which showed Alecensa achieving a remarkable 76% reduction in the likelihood of disease recurrence or death in patients with resected ALK-positive NSCLC, bolstered the marketing authorisation submission.
"For the first time, individuals in Europe who have undergone surgical resection for ALK-positive NSCLC have access to an ALK inhibitor, which can considerably lower the risk of disease recurrence or death," said Levi Garraway, M.D., Ph.D., Roche’s Chief Medical Officer and Head of Global Product Development.
"Surgery alone may not suffice for early-stage ALK-positive NSCLC, as the high risk of recurrence continues to trouble patients about their future," remarked Professor Fabrice Barlesi, a thoracic oncologist at Paris Saclay University and CEO of Gustave Roussy Institute. "The ALINA study revealed an unparalleled and consistent benefit in disease-free survival across all stages of the disease."
Alecensa is the treatment of choice for patients with advanced ALK-positive NSCLC and has revolutionized outcomes for those afflicted by this illness. It is approved as a first- and second-line treatment in over 100 countries, with more than 94,000 patients treated with Alecensa in clinical settings. With its approval for adjuvant treatment, Alecensa could, for the first time, be crucial for resectable ALK-positive disease, addressing a significant unmet medical need.
Alecensa is a highly selective, CNS-active, oral treatment developed at Chugai, part of the Roche Group, Kamakura Research Laboratories, for individuals with non-small cell lung cancer whose tumors are ALK-positive.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
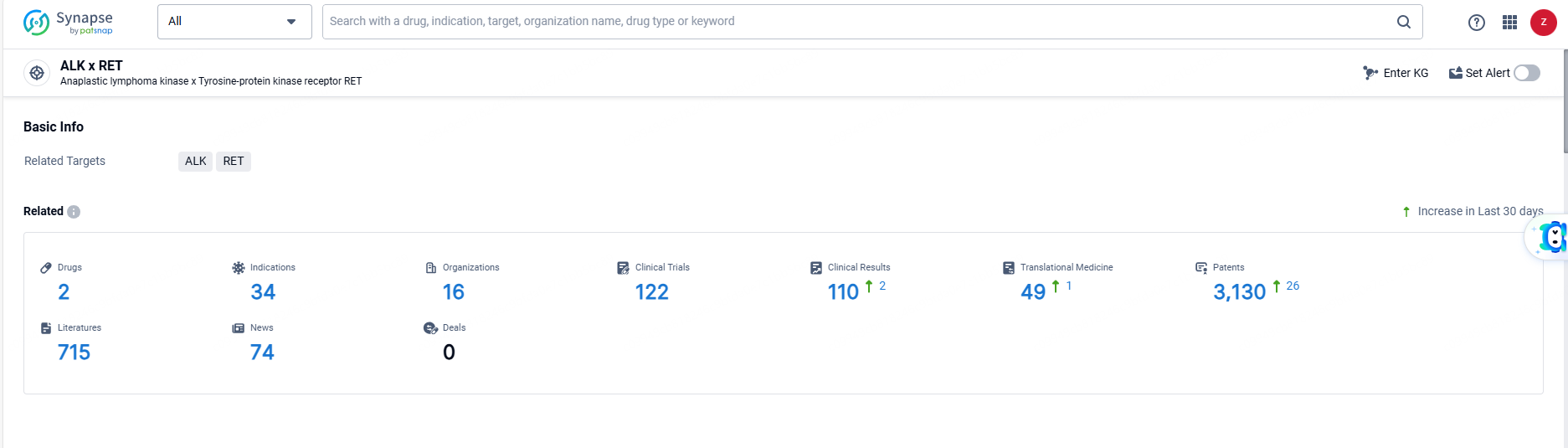
According to the data provided by the Synapse Database, As of June 14, 2024, there are 2 investigational drugs for the ALK and RET target, including 34 indications, 16 R&D institutions involved, with related clinical trials reaching 122, and as many as 3130 patents.
Alectinib Hydrochloride targets ALK and RET. It has been approved for the treatment of a wide range of therapeutic areas and indications, with its first approval in Japan in 2014. The drug has received regulatory designations and has reached the highest phase of development, indicating its efficacy and safety for use in patients.