FDA Approves Almirall's Klisyri® for Treating Actinic Keratosis on Face or Scalp
Almirall, an international pharmaceutical firm focused on medical dermatology, revealed that the U.S. Food and Drug Administration has given the green light to Almirall's latest supplemental New Drug Application, allowing an expanded application area for its medication, Klisyri, up to 100 cm2. Klisyri, an ointment that inhibits microtubules, is now authorized in a 350 mg packaging format and is indicated as a 5-day topical field therapy for actinic keratosis on the face or scalp.
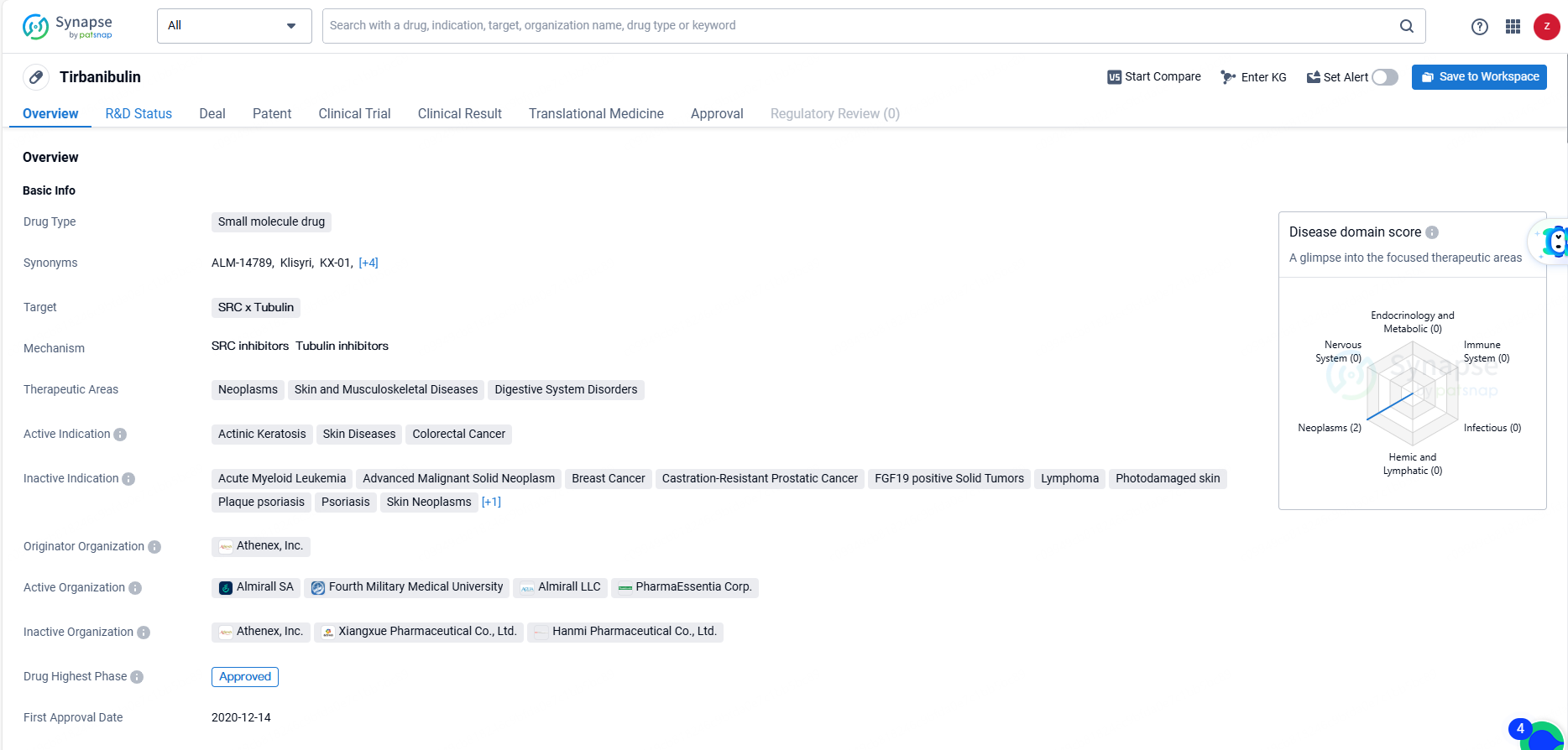
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"The FDA's authorization for extending Klisyri usage to treat actinic keratosis over a broader expanse of the face or scalp marks a major advancement for patients and dermatologists alike. As patients frequently present with AK affecting larger areas, dermatologists are striving to address the whole impacted region to avert the development of additional lesions," comments Karl Ziegelbauer, Almirall's Chief Scientific Officer.
With this new approval, the permitted dose of Klisyri (tirbanibulin) for surface area treatment has increased from 25 cm² to 100 cm², enabling healthcare providers to manage more extensive areas of the face or balding scalp.
The supplemental New Drug Application (sNDA) was backed by a further Phase 3, multicenter, open-label clinical safety trial involving over 100 participants in the US. The primary goals of the study were to assess the safety and tolerability of applying tirbanibulin to a field approximately 100 cm² in size on the face or balding scalp of adults with AK. The findings were consistent with the initial pivotal trials on a 25 cm² area, regarding both local skin reactions and treatment-related adverse events.
Tirbanibulin's efficacy in a larger treatment area was also investigated, showing a reduction in AK lesion counts similar to those observed in the initial pivotal studies.
"This new FDA approval grants clinicians the capability to treat up to four times the previous surface area, providing greater flexibility to manage actinic keratoses effectively while maintaining a favorable safety and tolerability profile for a broader patient population," states Dr. Neal Bhatia from San Diego, CA, the principal investigator for the pivotal study on a larger treatment area.
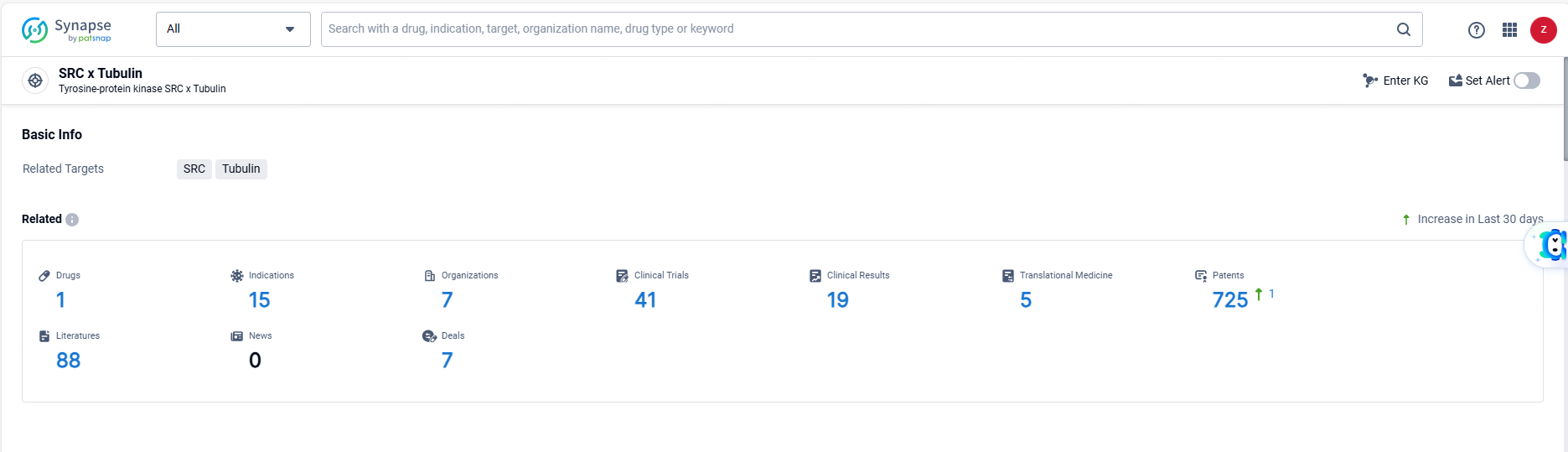
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of June 13, 2024, there are 1 investigational drugs for the SRC and Tubulin targets, including 15 indications, 7 R&D institutions involved, with related clinical trials reaching 41, and as many as 725 patents.
Tirbanibulin targets SRC and Tubulin. It is primarily used in the treatment of neoplasms, skin and musculoskeletal diseases, and digestive system disorders. The drug is indicated for actinic keratosis, skin diseases, and colorectal cancer. Tirbanibulin is designed to target specific proteins within cells, making it a promising option for the treatment of various diseases. Its approval for actinic keratosis and skin diseases indicates its potential in dermatological applications, while its use in colorectal cancer suggests its efficacy in treating certain types of cancer.